The energy change in a chemical reaction is the difference in the amount of the stored chemical energy between the products and the reactants.
The heat content of the system is known as enthalpy (which will be discussed in detail later) as energy is changed from one form to another because it can neither be created and nor be destroyed.
In chemistry, the chemical reactions involve energy changes such as during boiling, heat energy is gained. This was all about the introduction of this topic.
Now, let’s discuss some important concepts in detail now.
Energy Changes
Exothermic changes:
In an exothermic reaction, the temperature of the surrounding increases because heat energy is released. Similarly, it can be said that heat energy is given out to the surrounding.
For example, when methane (CH4) reacts with oxygen (in a process known as combustion), carbon dioxide and water are produced and heat energy is released.
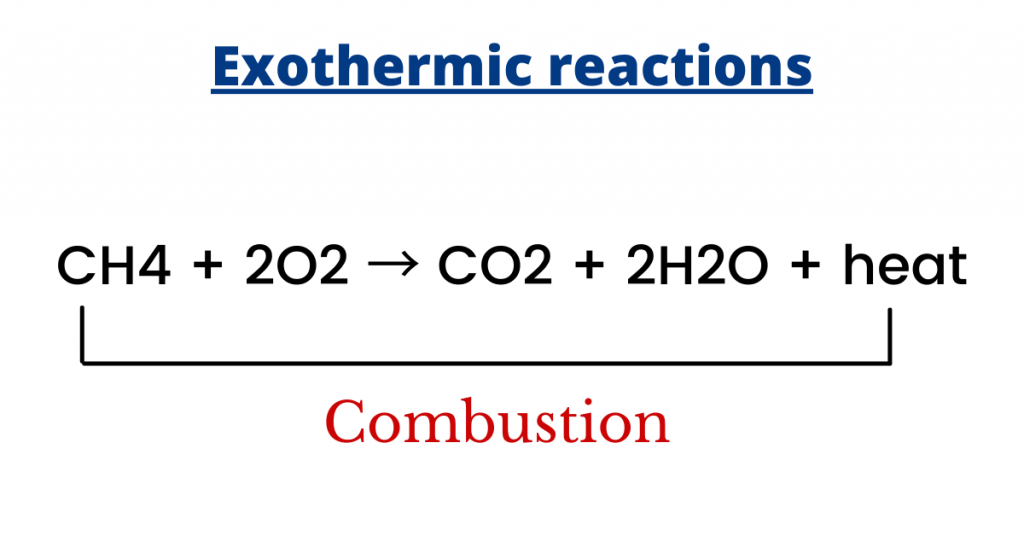
Furthermore, the condensation of water vapours into rain is an exothermic change since heat energy is given out to the surrounding.
As a result, the container in which the experiment is carried out will become warmer because the reaction mixture rises. Now you might be wondering, why is heat energy released?
The answer to this is simple. During bond making (the formation of bonds), the particles want to come together in order to form new bonds. As a result, they release heat energy and come closer to each other.
Some examples of exothermic processes, physical and chemical, are listed below:
- Freezing (physical)
- Condensation (physical)
- Respiration (chemical)
- Corrosion of metals (chemical)
Endothermic reactions:
The physical and chemical reactions are not bound to release heat energy (as in exothermic reactions). In endothermic reactions, heat energy is absorbed from the surrounding.
For example, plants absorb heat energy (from sunlight) during photosynthesis to produce glucose and oxygen from carbon dioxide and water.
Similarly, when you cook an egg, heat energy is absorbed from the pan. Since heat energy is absorbed, the surrounding temperature falls (decreases).
In other words, heat energy is transferred to the reactants (after it is absorbed) which causes the temperature of the reaction mixture to decrease.
As a result, the container in which the experiment is carried out will become cooler. Melting, evaporation, vaporization and sublimation are some physical processes that involve endothermic changes.

Likewise, thermal decomposition and photosynthesis are some chemical processes that involve endothermic changes.
This takes us to another very important concept in this topic which is about bond making and bond breaking in relation to exothermic and endothermic changes.
Bond making and bond breaking:
When bonds between atoms are formed, heat energy is released. Therefore, bond making is an exothermic process because heat energy is released during exothermic processes.
However, bond breaking is an endothermic process because heat energy is absorbed in order to break the bonds between the atoms. To find whether the reaction is exothermic or endothermic, find out the difference between energy gained during bond breaking and the energy released during the bond making.
If more energy is released than gained then, the reaction is exothermic while if more energy is gained than released then, the reaction is endothermic. Mathematically, it can be said that:
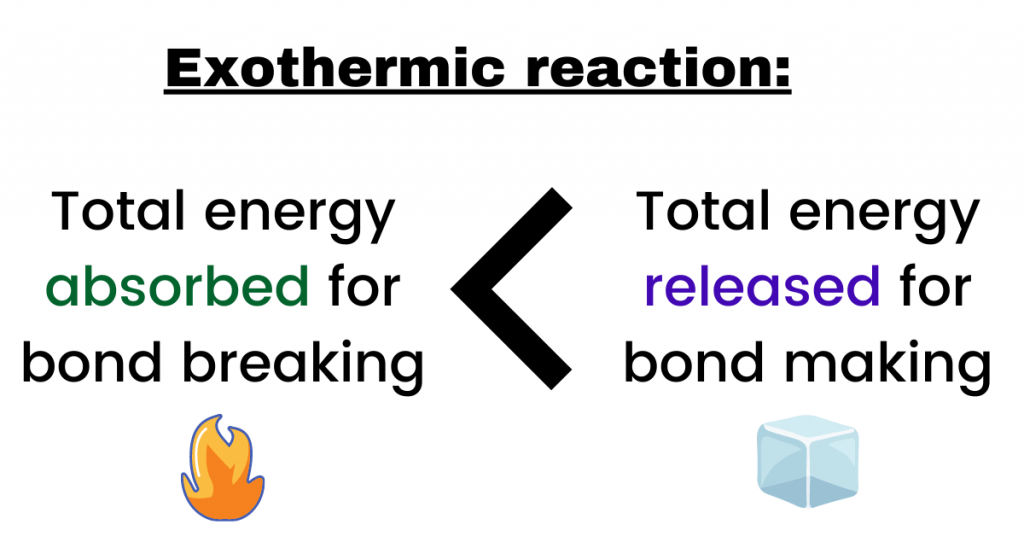
The amount of energy that is involved in a chemical reaction is known as the enthalpy change (ΔH). In other words, it is the difference (of energy) between the reactants and products. Therefore, it can be said that:
ΔH = energy absorbed (for bond breaking) – energy released (for bond making)
From the information above, we can say that if the reaction is exothermic, its enthalpy change (ΔH) will be in negative and note that enthalpy change is measured in kilojoules.
However, if a reaction is endothermic, the enthalpy change (ΔH) will be in positive because the total energy absorbed for bond breaking will be greater than the total energy released during the bond making.
Further reading:
Redox reactions | GCE O level notes
Deformation notes | GCE O level
In order to fully understand these above-mentioned concepts, let’s take an example. Find out whether the reaction below is endothermic or exothermic based on the given information.
2H2 + O2 → 2H2O
In the reaction above, the heat energy absorbed for bond breaking is 15000 KJ while the heat energy released during bond formation is 18000 KJ. What type of energy change does occur in the reaction?
Exothermic reaction because the enthalpy change will be as follows:
ΔH = 15000 – 18000 = -3000 KJ
Since ΔH is negative, the above conclusions can be made about the reaction. Now, let’s discuss the energy profile or energy level diagrams and learn some key concepts in them.
Energy profile diagrams:
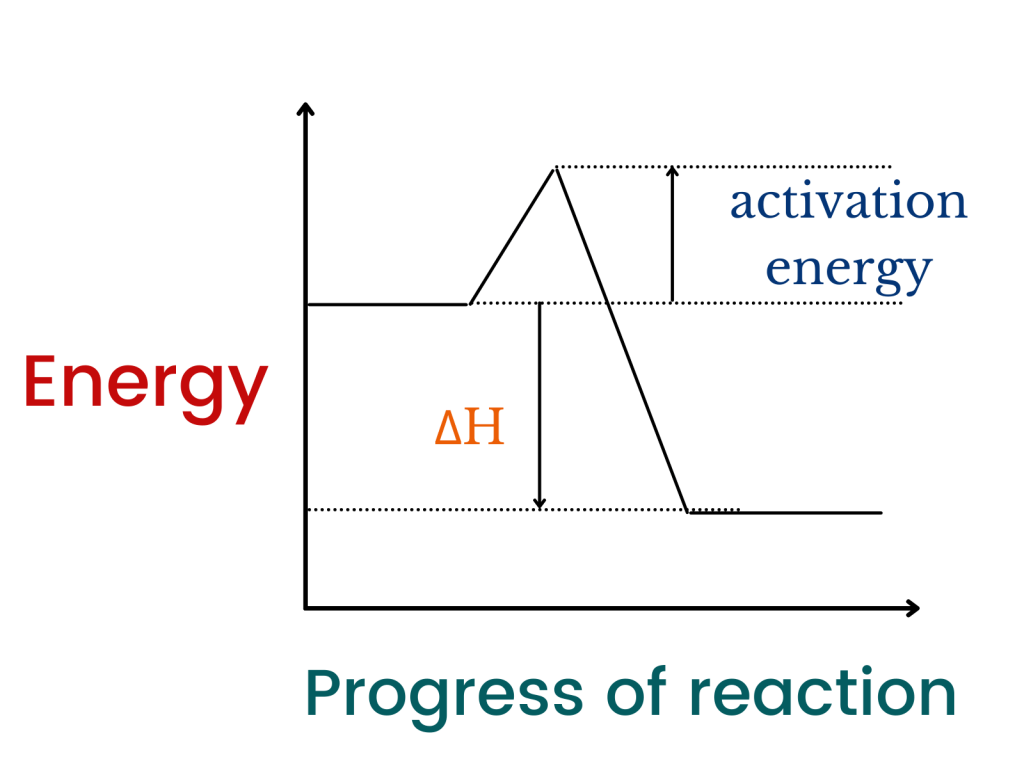
Energy level diagrams are a method which we use to show energy changes in a reaction. For exothermic reactions, the total energy of reactants is more than the energy of products. But, how can we say that?
Look, heat energy is released and it is only possible if the energy of reactants is greater than the energy of products. You can also remember that the enthalpy change will be negative in this case.
Energy profile diagrams do not only show the enthalpy change but also the activation energy (energy required by a reaction to proceed). Activation energy is the minimum amount of energy required by the reacting molecules to be converted into products.
In the energy level diagram for a exothermic reaction, the:
- reactants will be above the products
- the energy released to form bonds will be greater than the energy gained to break the bonds between the atoms
The diagram below shows the energy profile diagram for a reaction in which heat energy is released (note than energy is decreasing because the heat is being released).
However, the energy profile diagram for an endothermic reaction will indicate an increase in energy because heat energy is absorbed from the surroundings.
Therefore, when you are drawing an energy level diagram for the reactions in which heat energy is gained, draw the reactants (on the left) below the products (on the right).
The enthalpy change will be positive in this type of reaction. We use diagrams because it is easier to define how the energy of chemicals changes with the progress of the reaction.
Let’s take a look at an example.
2HF → H2 + F2
The reaction above is endothermic because energy absorbed for bond breaking is greater than the energy released for bond making. If we want to represent this reaction with the help of an energy profile diagram, we would do it as follows:
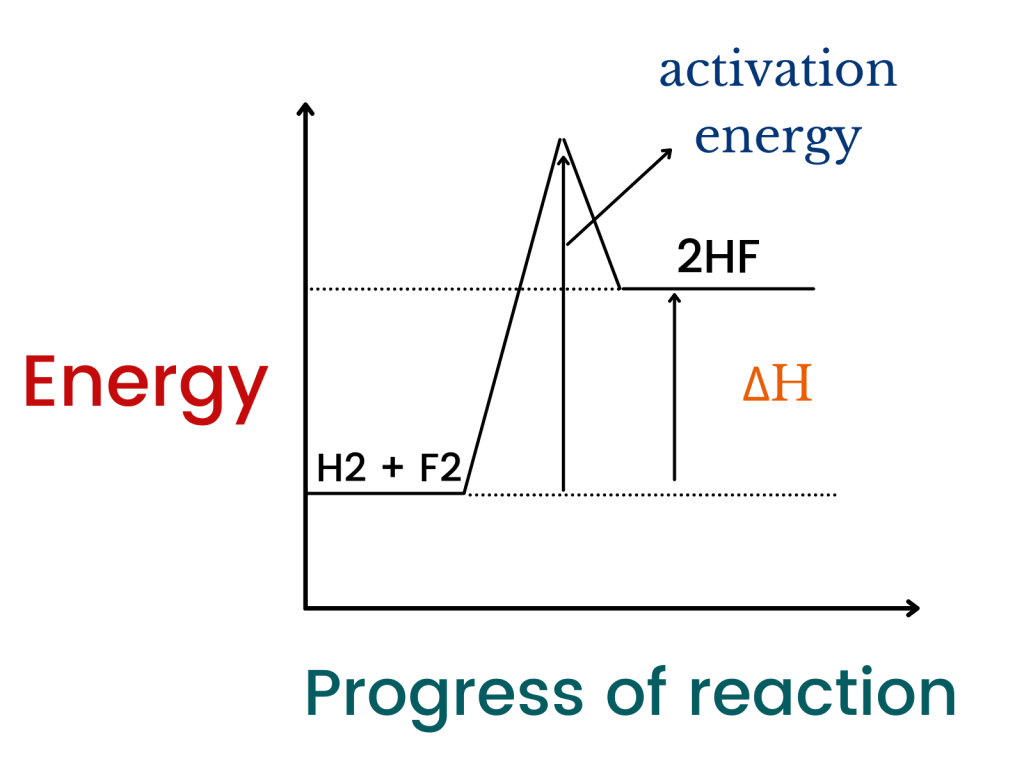
The diagram above demonstrates how the energy of chemicals changes during the reaction as it progresses. In this topic, you should also learn about fuel cells briefly.
Combustion reactions (burning in the air or reacting with oxygen gas) allow fuels (such as natural gas, wood and petroleum) to give out energy. Since energy is released, these reactions are exothermic.
Incomplete combustion refers to the limited supply of air due to which carbon does not burn completely to produce carbon dioxide gas. As a result, carbon monoxide, a harmful (poisonous) gas is produced.
Fuel cells:
Fuel cells are the cells that convert chemical energy into electrical energy and they resemble a battery in many ways. They are constantly provided with fuel and air are they are used in space projects.
Now, let’s talk about the woking of a fuel cell.
We have an anode (positive electrode) that provided electrons to the cathode (negative electrode) and these electrodes are immersed in an electrolyte (to conduct electricity – electrolysis).
Mostly hydrogen is provided to the anode where it is oxidised and hydrogen ions are produced. Oxygen is provided at the cathode where hydrogen ions gain electrons and react with oxygen to produce water.
The difference in the energy levels of the electrodes produces a voltage. Some of the advantages of fuel cells are:
- Greater reliability (power provided does not degrade)
- Reduces emission of greenhouse gases
- Environmental quality improves
- Demand for foreign oils reduces
- Fuel cells are compact and significantly lighter
However, some disadvantages of fuel cells are:
- Hydrogen is expensive and not widely available
- Lack of infrastructure for the development
- Fuel cells are costly and difficult to manufacture
- Hydrogen is very explosive and flammable
Practise Question:
Which of the following statements explain that the reaction is endothermic?
A. More energy is released during bond breaking than is absorbed when making product bonds.
B. More energy is gained during bond breaking than is released during the bond making.
C. Less energy is absorbed during bond breaking than is released during the bond making.
D. Less energy is released during bond breaking than is absorbed during the bond making.
The correct answer is B. Recall, that energy is released during the bond making, and is gained when breaking product bonds.
Conclusion:
With this, the article about energy changes has come to an end. I hope that all your questions have been answered and all queries have been cleared.
We discussed exothermic reactions, endothermic reactions, bond making, bond breaking and fuels cells above. You should carefully read the examples to completely understand the topic.
Practice plenty of past paper questions to ace this topic in the examination because it is very important from the paper point of view. Thank you very much for reading and staying with me till the end.
