Organic chemistry is a very important topic and there are multiple concepts that need to be addressed in this topic.
The topics that will be explained in this article are referred below so that you could consult them.
- Organic compounds
- Homologous series
- Functional group
- Alkanes
- Alkenes
- Alcohols
- Carboxylic acids
- Macromolecules (Nylon and Terylene)
Before we start with our main topic, I would like to tell you what exactly Cambridge wants you to know about this chapter. Therefore, the syllabus is listed below as well for your understanding (the expression has been changed for your understanding).
Organic chemistry syllabus (content and learning outcomes):

- Alkanes
- Describe homologous series in terms of the general formula, physical properties (melting and boiling point) and chemical properties
- Describe CnH2n+2 as the general formula of Alkanes and saturated hydrocarbons
- Draw the structures of alkanes (branched and unbranched), C1 to C4 and name the unbranched alkanes (methane to butane)
- Recognize isomers and define isomerism
- Alkenes
- Describe alkenes (unsaturated hydrocarbons) with a general formula of CnH2n and C=C functional group
- Draw the structure of alkenes (unbranched and branched), C2 to C4, and name the unbranched alkenes from ethene to butene
- Describe cracking hydrocarbons as a method to manufacture alkene
- Describe the difference between unsaturated and saturated hydrocarbons in reference to their structures and reaction with aqueous bromine
- Describe addition reactions of alkenes with steam, bromine and hydrogen and the properties of alkenes in terms of polymerization and combustion
- Describe the meaning of “polyunsaturated” when applied to food products and describe the manufacture of margarine by adding hydrogen to vegetable oils
- Alcohols
- Describe alcohols as a homologous series with a functional group of –OH
- Name the unbranched alcohols (methanol to butanol) and draw the structures of alcohols, C1 to C4
- The properties of alcohols in terms of oxidation and combustion
- The formation of ethanol by fermentation of glucose and the addition (catalysed) of steam to ethene
- State some advantages (uses) of ethanol
- Carboxylic acids
- Describe the functional group of carboxylic acids –COOH
- Name the unbranched acids, methanoic to butanoic acids, and draw the structures of carboxylic acids (methanoic to butanoic acids)
- Describe the reaction of carboxylic acids (weak acids) with metals, carbonates and bases
- The oxidation of ethanol by potassium magnate (VII) to form ethanoic acid and the making of vinegar by bacterial oxidation
- Describe the reaction between alcohols and carboxylic acids to form esters
- Draw the structures of ester, name them and state some commercial uses
- Polymers
- Describe polymers (large molecules) that are made from monomers (small units)
- The formation of poly(ethene) from the addition polymerization of ethene (monomer)
- State the uses (commercial benefits) of poly(ethene)
- Describe condensation polymerization in terms of Nylon and Terylene (condensation polymers)
- Learn to deduce the structure of polymer from monomer and monomer from polymer
- Describe carbohydrates and proteins as natural polymers
- Describe that Nylon and proteins have the same amide linkage
- Describe that fats and Terylene have the same ester linkage
Alkanes:
Alkanes are the homologous series of organic compounds that contain carbon and hydrogen atoms which are single-bonded.
Alkanes have no other functional group and their general formula is CnH2n+2.
What is a homologous series? What do you mean by general formula? What is a functional group? It is time to explain to you these terms.
Homologous series refers to the families in which the organic compounds are grouped into.
Therefore, the compounds in a homologous series have the same general formula and similar chemical properties. Alkanes, alkenes, alcohols and carboxylic acids are the examples of homologous series.
There are multiple charactersticks of a homologous series such as they have similar chemical properties, same functional group and a change in their physical properties (going down the series).
Similarly, the functional group refers to an atom (or group of atoms) that provide a molecule with its characteristic properties. You can refer to the characteristic properties as chemical reactions.
The composition of a compound is shown by the general formula which is basically a type of empirical formula. I hope that these terms make sense to you now.
How do we name Alkanes?
You should remember that the name of all alkanes end with “-ane“. The molecular formula of alkanes is derived from their general formula which has been discussed earlier.
The name and molecular formula of some alkanes are mentioned below for your reference as well.
Methane (CH4)
Ethane (C2H6)
Propane (C3H8)
Butane (C4H10)
You should also know the structural formula of the above-mentioned alkanes (the structural formula of ethane is showed below).
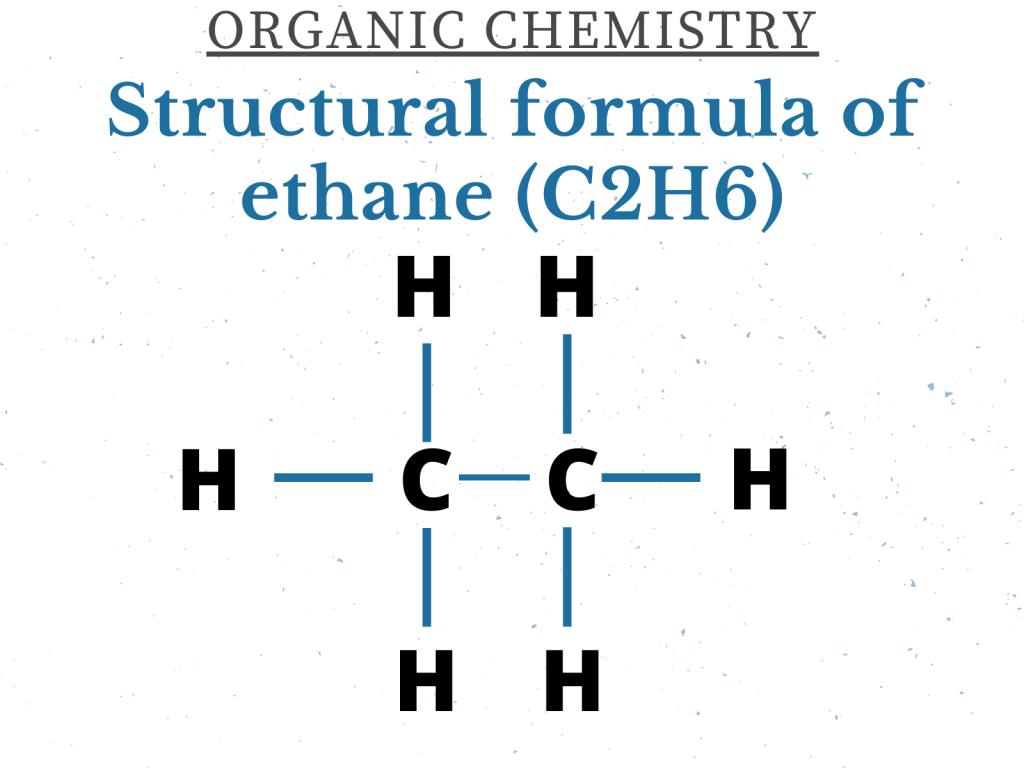
Alkanes are saturated hydrocarbons because there is Carbon-Carbon single covalent bond in them. The carbon atoms are bonded to hydrogen atoms as well.
These atoms can bond with four atoms (maximum) and therefore, they are known as saturated hydrocarbons. Saturated hydrocarbons are the simplest class of hydrocarbons.
It is time to move to another important concept about alkanes which is about the isomerism in alkanes. So, let’s discuss that topic as well.
Isomerism in Alkanes:
The compounds which have a similar molecular formula but different structural formula (arrangement of atoms) are known as isomers.
Isomers have different physical properties as well such as their melting point and boiling point. You should also know how do we name isomers.
Isomers are named in accordance with their structural formula (we use the side chain to determine the name of the isomers). The side chain is the alkyl group and the examples of the alkyl group are:
- Methyl: CH3-
- Ethyl: CH3 CH2-
- Propyl: CH3 CH2 CH2-
- Butyl: CH3 CH2 CH2 CH2-
Let me explain this topic to you with the help of an example as well.
In the image below, we have the same molecular formula (C4H10) but the structural formula (arrangement of the atoms) is different.
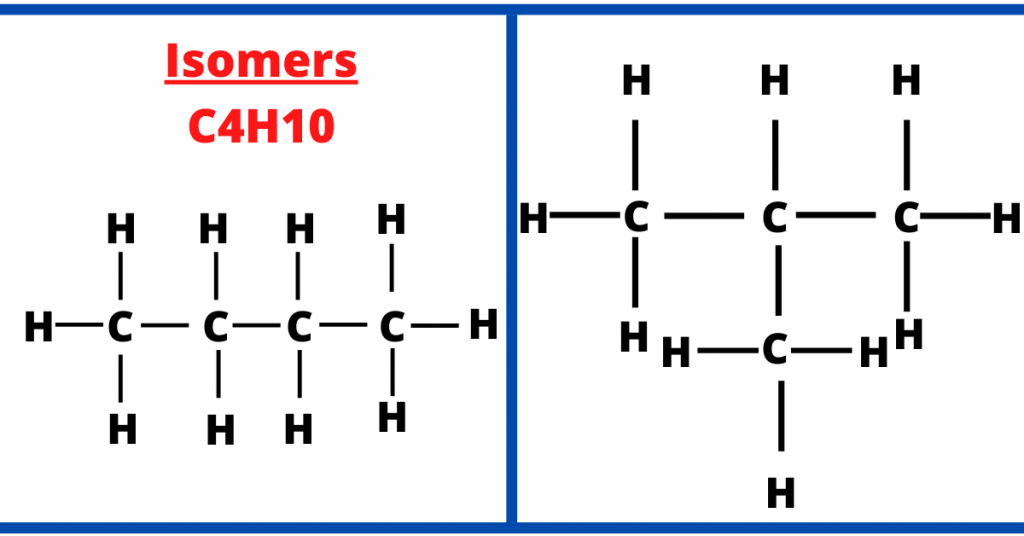
This is because Butane (in the example) is a straight-chained alkane while Methypropane has a side chain of -CH3. Therefore, it is said to be a branched alkane.
As a result, the compounds are the isomers of each other and this is an example of isomerism in alkanes.
This takes us to another important topic which is about the properties of alkanes.
Properties of Alkanes
Physical properties:
- Alkanes are colourless
- They are soluble in organic solvents
- They are insoluble in water
- Their melting and boiling point is low
Going down the series, how are the physical properties of alkanes affected?
- The melting and boiling point increases down the series (of alkanes).
For example, the melting and boiling point of ethane is lower than that of butane. This is because the forces of attraction between the molecules of butane are stronger as the molecules are bigger in size as compared to ethane.
- Down the series, alkanes become less flammable because the carbon content increases and they become more viscous (flow less easily).
Chemical properties of alkanes:
- Alkanes react with halogens (group 7 of the periodic table), such as chlorine and bromine, for the substitution reactions to occur.
These reactions occur in the presence of ultraviolet (UV) light. For example:
CH4 + Br2 → CH3Br + HBr
In the above example, one hydrogen atom is replaced (in methane) by a bromine atom for the substitution reaction to occur. In this way, further hydrogen atoms can also be replaced by bromine atoms.
In simple words, the functional group of a chemical compound is replaced by another functional group in a substitution reaction.
- Alkanes react with oxygen to produce carbon dioxide and water vapour. The balanced equation of this reaction is (in case of ethane)
2C2H6 + 7O2 → 4CO2 + 6H2O
This chemical property shows why methane (an alkane) is highly flammable and as a result, it is used for cooking purposes as well.
These are some of the chemical properties of alkanes which Cambridge requires you to know. I have elaborated these properties with examples so that you can easily understand this topic.
Now, we are going to cover the topic of alkenes.
Alkenes:
Alkenes are the class (series) of hydrocarbons (contain carbon and hydrogen) that contain at least one carbon-carbon double covalent bond.
Alkenes are unsaturated hydrocarbons. This means that they have double covalent bonds between their carbon atoms. Since these compounds can add more hydrogen atoms, they are known as unsaturated hydrocarbons.
In simple words, alkenes contain less than maximum possible hydrogen atoms which make them unsaturated. I hope that this will be easy enough to comprehend.
The general formula of alkenes is:
CnH2n
When talking about alkenes, we also have to understand the topic “Isomerism in alkenes”. The term “isomerism” and isomers have been discussed earlier.
Further reading:
Now we will take a look at isomerism in alkenes.
Isomerism in alkenes:
As mentioned earlier, isomers have the same molecular formula but different structural formula (arrangement of atoms) which make them isomers of each other.
Let me give you an example to elaborate this topic.
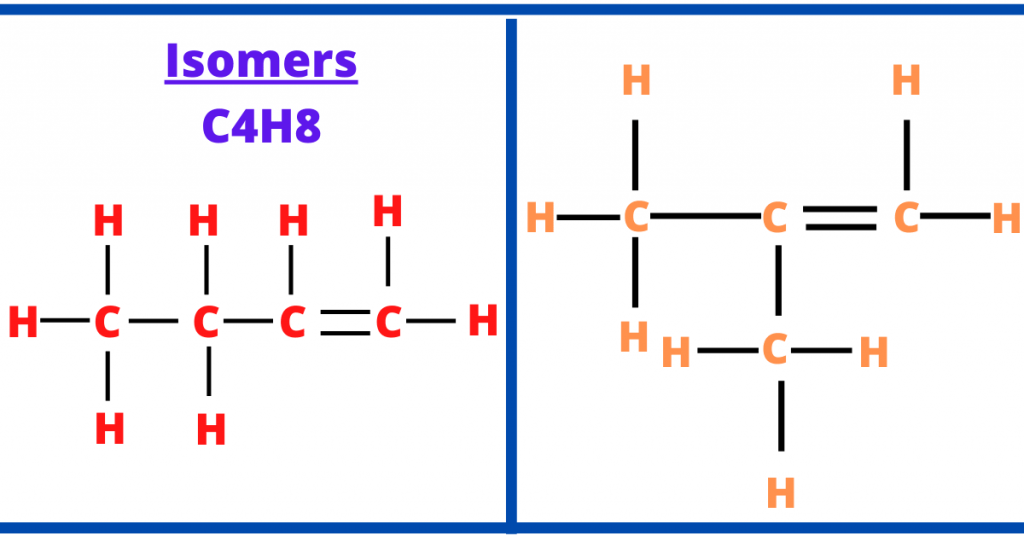
In the image above, you can clearly see that the molecular of the two structures are same. The molecular formula is C4H8.
However, the structural formula of these compounds is different because butene (represented in red in the diagram) is a straight-chain alkene while methylpropene (represented in orange in the diagram) is a branched alkene.
Moreover, these two compounds have different melting and boiling point and you should know that isomers have different melting and boiling point.
This is all about isomerism in alkenes. So, it is time to move to another important topic which is about the properties of alkenes (physical and chemical).
Physical properties of alkenes:
- Alkenes are colourless and odourless (except ethene which has a sweet odour) in nature.
- Ethene, propene and butene are gases at room temperature.
Note: Not all alkenes are gases at room temperature. Some alkenes are liquid while some are solids as well!
- They are insoluble in water
- The boiling point of alkenes increases with an increase in the (number of) carbon atoms.
These are not all the physical properties of alkenes but these are enough to meet your syllabus requirements and for general knowledge as well.
Chemical properties:
- Alkenes react with oxygen (which means that they burn in excess air) to form carbon dioxide and water vapour.
Let me use ethene as an example to explain this.
C2H4 + 3O2 → 2CO2 + 2H20
As you can see in the equation above, ethene (an alkene) reacts with oxygen to produce carbon dioxide and water vapours. This is because the carbon atoms in the alkene molecule undergo combustion reaction.
- Alkenes react with hydrogen to form alkanes but there are some requirements for this reaction to occur.
This reaction occurs at 200°C and in the presence of Nickel (which acts as a catalyst in this reaction).
For example
propene + hydrogen → propane
C3H6 + H2 → C3H8
This process is also known as hydrogenation and it is very useful in our daily life. For example, vegetable oil undergoes this reaction to form margarine.
- When an alkene is added to bromine solution, the reddish-brown colour changes immediately into colourless (decolourises).
This reaction is known as bromination.
Well, this was some basic knowledge about bromination but what is really important over here is that this reaction can be used as a test to differentiate between alkanes and alkenes.
Alkanes (saturated hydrocarbons) do not decolourise (turn into colourless) the bromine solution while alkenes (unsaturated hydrocarbons) decolourise bromine into a colourless liquid.
- Alkenes react with steam to produce (form) alcohols.
This is one of the most important reaction you should know about. Similarly, there are certain conditions which are required for this reaction to happen. These are mentioned below.
This reaction occurs at around 300°C and phosphoric (V) acid (H3PO4) is the catalyst which is used in this reaction. For example,
ethene + steam → ethanol
C2H4 + H2O → C2H5OH
Organic chemistry requires you to remember that this is one of the methods that we use to produce alcohols. Another method is the fermentation of glucose which will we study later in this article so stay tuned because it is very important.
This takes us to another very important topic which is about cracking.
Manufacturing alkenes by cracking hydrocarbons:

Cracking is a process in which long-chain hydrocarbons are broken down into smaller (and useful) molecules. We obtain alkenes by cracking crude oil (petroleum).
We can use a catalyst (a biological substance to speed up a chemical reaction) to speed up this process, cracking, and therefore, it is also known as catalytic cracking.
In organic chemistry, you should know that a very high temperature is required for this reaction to occur and silicon dioxide (SiO2) and aluminium oxide (Al2O3) are the catalysts for cracking.
Cracking is very important because it provides us alkenes (short-chain) that are very useful. For example, ethene can be used to manufacture ethanol (alcohol) and propene for making plastics.
Moreover, cracking is also used to produce hydrogen and petrol.
Polyunsaturated refers to more than one carbon-carbon double (covalent) bond.
This is all about alkenes you should know.
Alcohols:
These (alcohols) are a homologous series of organic compounds that have a -OH (hydroxyl) functional group.
Alcohols have the general formula CnH2n+1OH and they contain three elements which are:
- Carbon
- Hydrogen
- Oxygen
You should remember that the name of all alcohols ends with ‘-ol‘ and some alcohols are mentioned below for your guidance.
Methanol (CH3OH)
Ethanol (C2H5OH)
Propanol (C3H7OH)
This was some basic knowledge about the topic and it is time to move to a very important topic which is about the properties (physical and chemical) of alcohols.
Physical properties of Alcohols:
- Their solubility decreases due to an increase in their molecular size
- These (alcohols) are volatile liquids at room temperature and pressure
- Their boiling point increases with an increase in their molecular size because the forces of attraction also become stronger.
Therefore, a larger temperature is required to overcome these forces of attraction.
Chemical properties of alcohols:
In the topic of alkanes and alkenes, we learned that they undergo combustion reaction to liberate carbon dioxide and water vapours.
- Similarly, alcohols also react with oxygen (combustion reaction) to produce carbon dioxide and water vapour. For example:
C3H7OH + 5O2 → 3CO2 + 4H2O (balanced equation)
This reaction is very useful in our daily life because it is used to give flavours to food items and therefore, alcohols can be used as fuels as well.
- Alcohols also undergo oxidation reactions when they are heated with an oxidising agent such as acidified potassium manganate (V||).
When this reaction occurs, carboxylic acid and water are formed (we will learn about carboxylic acid later in this article). Let me explain this reaction with the help of an example.
C2H5OH + 2[O] → CH3COOH + H2O
In the reaction above, ethanol (alcohol) reacts with oxygen from an oxidising agent, which is represented by [O], to produce ethanoic acid and water.
This was an example to illustrate oxidation reactions in alcohols. This reaction is very important because it is used for various purposes such as to manufacture cleaning products in industries.
In the topic of alkenes, we learned that the reaction of alkenes with steam produces alcohols. Well, you should also know that the fermentation of glucose is another method to produce ethanol.
Fermentation to produce ethanol:
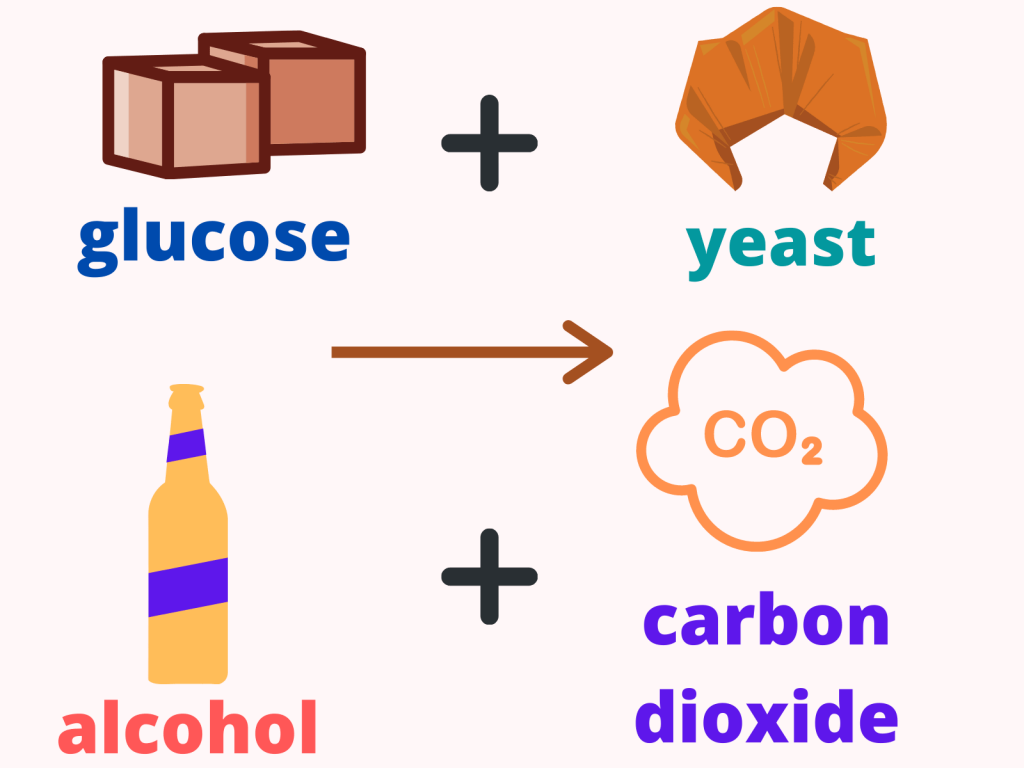
Ethanol is present in alcoholic drinks such as beer and wine and to produce ethanol, we ferment things such as vegetables and fruits.
In this process, we will use micro-organisms, such as yeast, to produce ethanol after reacting with carbohydrates (like glucose) in the absence of oxygen.
Note: Carbon dioxide is also produced during this reaction.
The equation for this reaction is listed below for your guidance.
C6H12O6 → 2C2H5OH + 2CO2
In the reaction above, a glucose solution (mixed with yeast) undergo fermentation to produce a dilute solution of ethanol.
Important note: The temperature for this reaction should be 37-degree celsius because, at this temperature, enzymes work best and this reaction only occurs in the absence of oxygen.
Ethanol is a good solvent, used in alcoholic drinks and is also used as a fuel. This was all about the topic of alcohols.
It is time to move to another significant topic in organic chemistry which is about carboxylic acids. So, let me explain this topic to you as well.
Carboxylic acids:
These (carboxylic acids) are the homologous series of the organic compound who have the -COOH (carboxyl) functional group. The general formula of carboxylic acids is CnH2n+1COOH.
In alcohols, I explained that the name of alcohols ends with ‘-ol’ such as methanol. The name of carboxylic acids ends with ‘-oic acid’. The name of some carboxylic acids has been shown below so that you can easily understand this topic.
- Methanoic acid (HCOOH)
- Ethanoic acid (CH3COOH)
- Propanoic acid (C2H5COOH)
- Butanoic acid (C3H7COOH)
Remember that the formula, written in brackets above, is derived from the general formula of carboxylic acids which is CnH2n+1COOH.
Physical and chemical properties of carboxylic acids:
- The boiling point of carboxylic acids increases with an increase in their molecular size.
This is because the electrostatic forces become stronger and to overcome them, a higher temperature is required.
- Methanoic acid, ethanoic acid, propanoic acid and butanoic acid are soluble in water.
These are some of the physical properties of carboxylic acids which you should know about.
You should remember that carboxylic acids are weak acids because they partially ionise in water (which means that they do not completely release all hydrogen ions in aqueous solution).
You should also learn the chemical properties of carboxylic acids (which are mentioned below).
- Carboxylic acids react with metals (such as sodium, magnesium and potassium) to give a salt and hydrogen. For example
2CH3COOH + 2Na → 2CH3COONa + H2
- Carboxylic acids react with bases to form salt and water
- Carboxylic acids react with carbonates to form salt, carbon dioxide and water. For example:
2CH3COOH + Na2CO3 → 2CH3COONa + CO2 + H2O
Lastly, carboxylic acids react with alcohols to form esters (sweet-smelling compounds).
What is an ester? Well, an ester is a colourless liquid which is insoluble in water and the process to form esters (reaction of carboxylic acids with alcohols) is known as esterification.
Concentrated sulphuric acid is used as a catalyst in this reaction.
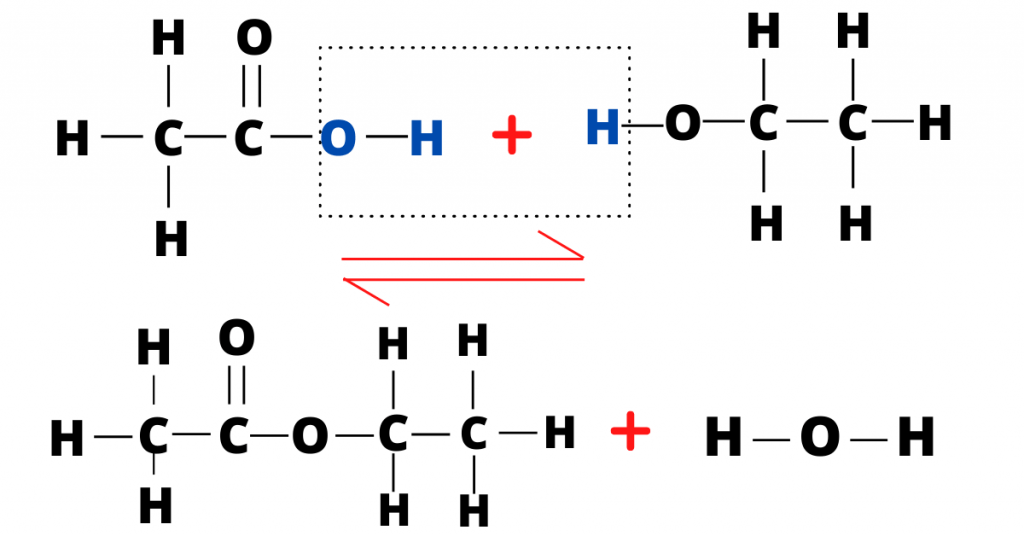
In the image above, you can see the reaction between ethanoic acid and ethanol to form the ester, ethyl ethanoate, and water.
You should also know how the name, ethyl ethanoate has been derived. Therefore, let me tell you how do we name esters.
The first part (name) of an ester is derived from the alcohol while the second part of the name is derived from the carboxylic acid.
For example, the reaction between ethanoic acid (carboxylic acid) and methanol will form an ester which will be called methyl ethanoate.
Note that methyl is derived from methanol while ethanoate is derived from ethanoic acid.
Moreover, esters are very useful in our daily life because they are used for the preparation of artificial flavouring. They are also used as solvents for perfumes and cosmetics.
Polymers (addition and condensation polymerization):
Polymers are the large molecules that are made from repeating units known as monomers. Polymers are also known as long-chain macromolecules.
Carbohydrates, proteins and silk are examples of natural polymers while nylon and terylene are synthetic (man-made) polymers. There are two reactions to form polymers and those are:
- Addition polymerization
- Condensation polymerization
In addition polymerization, unsaturated monomers join together to form an addition polymer. In this reaction, no atom or molecule is lost.
For example, the addition polymerization of ethene to form poly(ethene) is shown below.
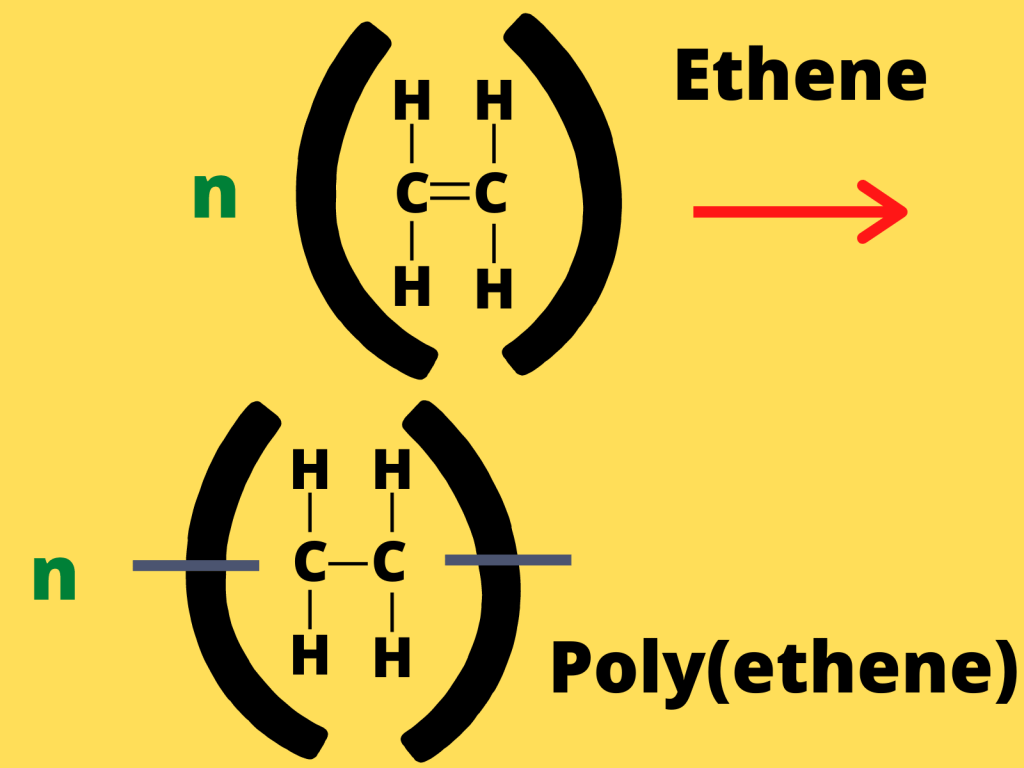
As you can see in the image, the carbon-carbon double covalent bonds of ethene break (due to high temperature and pressure). The n indicates multiple monomers. When these monomers break, they combine to form poly(ethene).
Addition polymerization (as discussed above) is very important because poly(ethene) is used to make various toys, plastic bags and various other instruments due to its properties.
Now let’s talk about condensation polymerization.
In condensation polymerization, small repeat units (monomers) combine to form a polymer and water is removed during this process.
Nylon and terylene are made due to condensation polymerization. Organic chemistry requires you to learn how monomers react to form nylon and terylene.
dicarboxylic acid + diamine → nylon + water
dicarboxylic acid + diol → terylene + water
It is to note that there is an amide linkage in the condensation polymerization to form nylon (because of the presence of diamine) while there is an ester linkage in the condensation polymerization to form terylene
Now, I will show you the condensation polymerization to form nylon. Therefore, you can consult the structure below for your guidance (note that the repeating amide links can be seen in nylon).
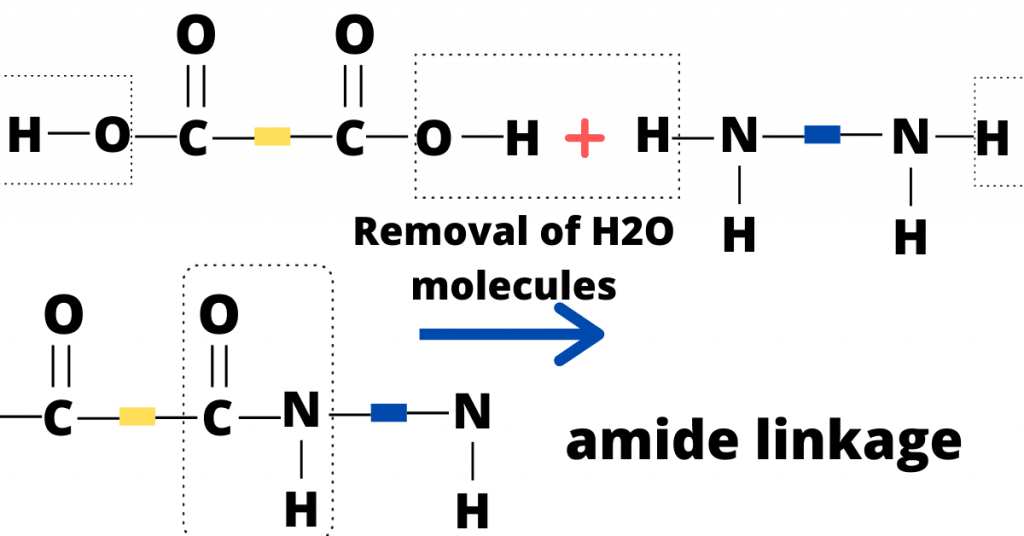
You should remember that nylon and proteins have the same amide linkage even though nylon is a synthetic polymer while proteins are natural polymers.
Similarly, terylene (a synthetic polymer) and fats (a natural polymer) have the same ester linkage which is visible in their structural formula.
Conclusion:
With this, our topic about organic chemistry notes has come to an end. I hope that all your queries have been addressed. If you have further questions, you can contact us.
This is a very important topic in chemistry and therefore, all the concepts have been explained in detail for your proper guidance. The topics that have been addressed in this article are:
- Alkanes
- Alkenes
- Alcohols
- Carboxylic acids
- Esters
- Macromolecules
You should practise plenty of past papers to ace this topic. Thank You very much for reading and staying with me till the end and stay tuned for more useful articles and resources.
