In chemistry, electrolysis is a method in which an electric current is passed through a substance to break it down (decomposition).
This method is carried out in an electrolytic cell (an apparatus comprising positive and negative electrodes that are placed in a solution of positive and negative ions).
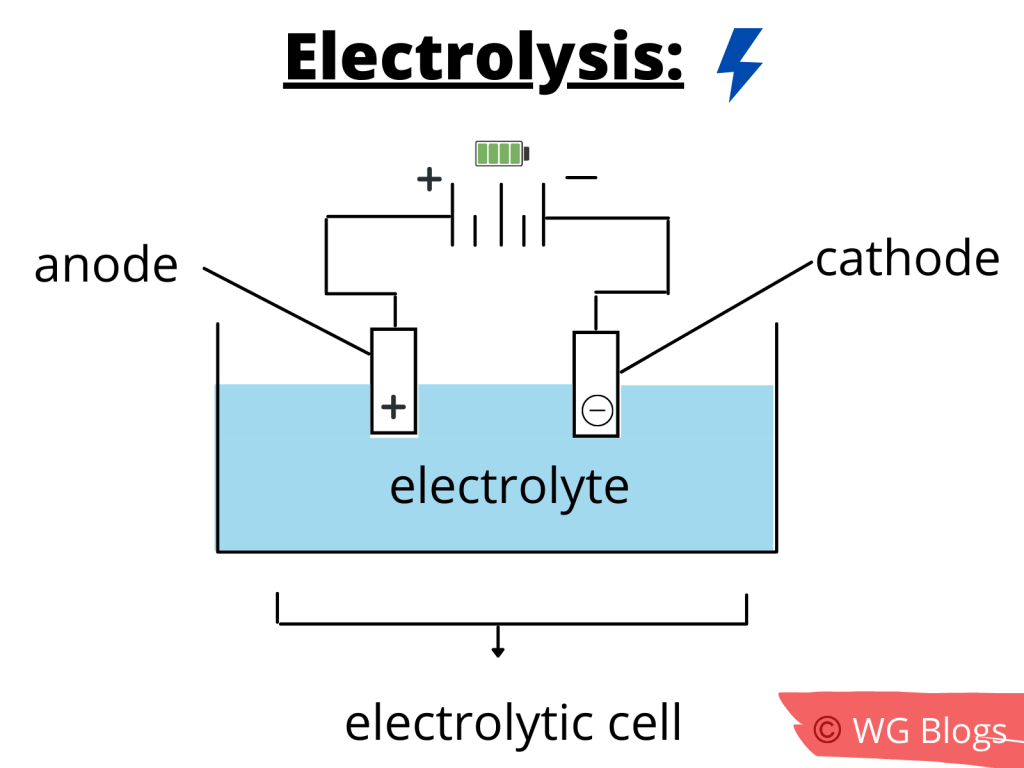
Note: Covalent compounds do not conduct electricity, so they do not undergo electrolysis. However, the aqueous solutions of ionic compounds do because they can conduct electricity.
“Decomposition potential” is the voltage required during this process and is used for multiple commercial purposes, such as extraction of pure metals from their ores.
Moreover, it is also used for the deposition of metals from the solution (known as electroplating). This concept of electroplating will be discussed later in this article.
This was all about the introduction of this topic. Now, let’s discuss some important concepts regarding the chapter as well in detail now.
The basics:
Before we dive deep into the topic, it is essential to go across some terms to prevent confusion in the future.
- Cation: It is a positively charged ion that is attracted to the cathode (negative electrode).
- Anion: It is the negatively charged ion that is attracted to the anode (positive electrode).
- Electrode: It is a good electrical conductor that connects the non-metallic parts of a circuit. For instance, an electrolyte. Simply, it is a part from which the electric current flows.
- Electrolyte: A substance (an ionic compound in molten or aqueous solution) that produces an electrically conducting solution. Simply, it conducts electricity (such as dilute sulphuric acid).
The substances that do not conduct electricity, such as ethanol, sugar, distilled water and sulfur are called non-electrolytes.
During the process, the battery provides electrons that flow from the positive to the negative terminal (in the battery).
Oxidation occurs at anode (positive electrode) because anions give up (lose electrons).
Reduction occurs at the cathode as cations gain electrons.
Let us take a look at some examples to improve our understandings of the topic.
Electrolysis of molten calcium chloride:
If we heat calcium chloride, it turns into a molten (melted) state where it has calcium (positive) and chloride (negative) ions.
When this compound is supplied with an electric current, the negative chloride ions move towards the anode (positive electrode) to be oxidised to form chlorine gas.
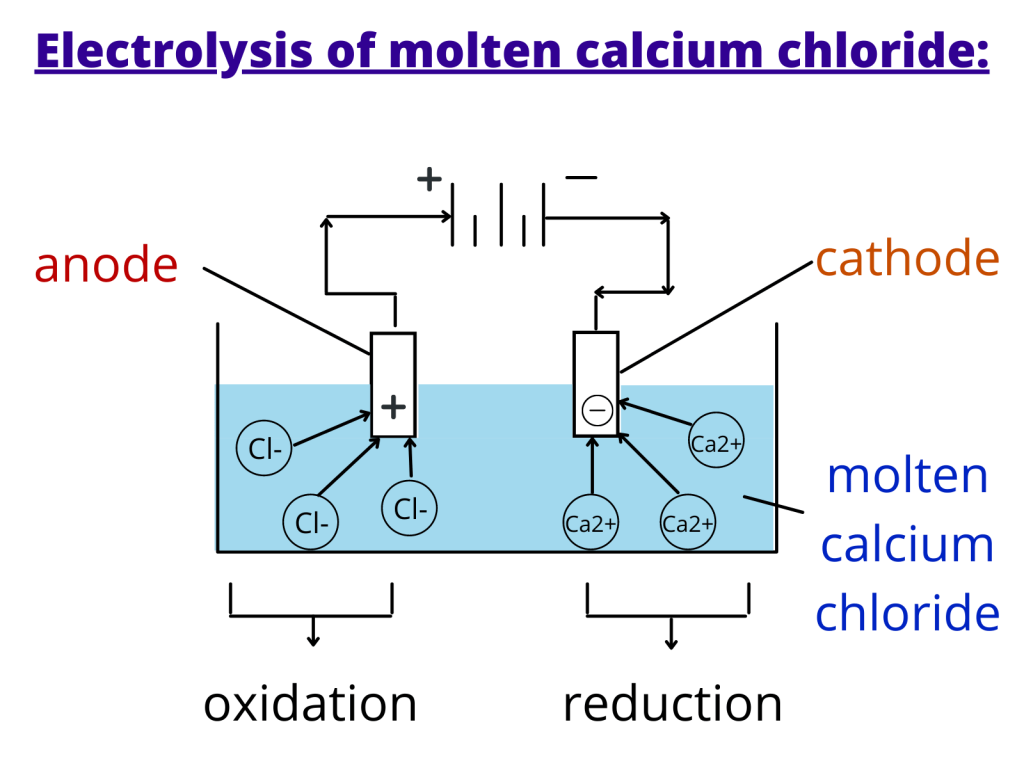
Similarly, the positive calcium ions will move towards the cathode where they will lose electrons (reduced) to form a metal that is deposited on the surface of the electrode (graphite in this case).
Note: Platinum and graphite (a form of carbon) are used to make Inert electrodes because do not react with the products and the electrolyte.
The equations for the reactions at both electrodes are:
At cathode:
Ca2+ + 2e- → Ca
At anode:
2Cl- → Cl2 + 2e-
Now, let’s discuss what will happen when molten lead (||) bromide will undergo the same reaction.
Molten lead (||) bromide:
When this compound will be supplied with an electric current, the positive lead ions will move towards the negative cathode.
However, the negative bromide ions will move towards the positive anode where they will lose electrons to form bromine gas (indicated by the bubbling at the electrode).
The lead ions will gain electrons (reduction) to form grey lead metal. The equations at both electrodes are:
At cathode:
Pb2+ + 2e- → Pb
At anode:
2Br- → Br2 + 2e-
Now, I want you to find out the products when the electrolysis of molten sodium chloride will take place. Remember, the ions will be sodium (positive ion) and chlorine (negative ion).
Done?
If you have figured out that sodium metal and chlorine gas will be the products then, you deserve due credit.
But if you failed to find out the correct answer, do not worry. If you follow me, you will understand this topic.
Electrolysis of aqueous solutions:
The solution in which the solvent is liquid water, that solution is known as an aqueous solution (the solute ions or molecules are surrounded by water molecules).
For instance, an aqueous solution of lead (||) bromide contains lead bromide and water. Therefore, the ions in this solution are:
- Pb2+ (aq)
- Br– (aq)
- H+ (aq)
- OH- (aq)
This shows that in an aqueous solution, there is more than one cation and anion. So, which moves towards the respective electrode?
To find this, we use the reactivity series.
We have the reactivity series (also known as activity series) for which we can find out the ease of discharge of both cations and anions
Normally, the ion with the greater ease of discharge will move towards the respective electrode because “ease of discharge” refers to the tendency to be discharged.
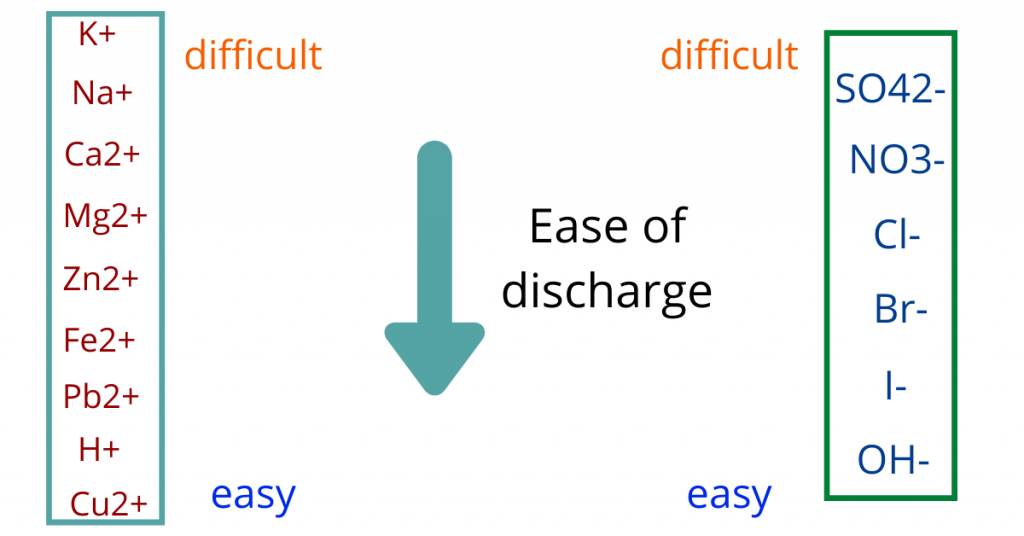
If this is confusing you, do not worry. We will take a look at some examples to further understand these concepts.
Aqueous Copper (||) Sulphate:
When water is added to copper (II) sulphate (CuSO4), it dissolves in water to form an aqueous solution. This solution has the following ions:
- Cu2+ (ion from copper sulphate)
- SO₄²⁻ (ion from copper sulphate)
- H+ (ion from water)
- OH- (ion from water)
The copper ions will be attracted towards the cathode because they are lower in the reactivity series than hydrogen. This means that the ease of discharge of copper ions is more than hydrogen ions.
On the cathode, the copper ion will gain two electrons to become a copper atom. The equation below shows the reaction at the cathode:
Cu2+ + 2e- → Cu
On the other hand, the ease of discharge of hydroxide ions (OH-) is more than sulphate ions because hydroxide ions are lower in the reactivity series.
Therefore, they will move towards the anode to form oxygen gas (because OH- ions give up electrons more readily than sulphate ions). The reaction at the anode is shown below:
4 OH- → 2 H2O + O2 + 4e-
Dilute sodium chloride:
When an electric current is passed through dilute sodium chloride (NaCl), we get hydrogen and oxygen gas as a result of this reaction.
How does this happen?
The ions from this solution are Na+, Cl-, H+, OH- (from sodium chloride and water). Now, which ion will move towards the respective electrode?
If we look at the ease of discharge, hydrogen is below sodium. Therefore, the tendency of hydrogen ion to gain electron is more than sodium ion.
This is the reason why hydrogen ion will move towards the cathode to be reduced (by gaining an electron).
Further reading:
Energy changes notes | GCE O Level
Oxidation and Reduction reactions
Apart, the hydroxide ion (OH-) will move towards the anode because if we look at the ease of discharge, the hydroxide ion is below chloride ion. This means that the hydroxide ion gives electrons more readily than chloride ions.
The equation at this electrode will be:
4OH- → 2H2O + O2 + 4e-
This shows how oxygen will be evolved at the anode.
Question: What will happen when an electric current will be passed through water?
We can use this process to decompose water as well into hydrogen and oxygen gas but this can be a little complicated. The reason being the non-conductivity of water.
As a result, we acidify water with dilute sulphuric acid or hydrochloric acid. Due to this, the bonds between the water molecules are broken. This allows electricity to be conducted.
This takes us to our next concept which is about concentrated solutions.
Electrolysis of concentrated aqueous copper chloride:
When we have a concentrated solution, things become a little bit different due to the effects of concentration on the anions.
Let me explain this with the help of an example.
Normally, we would say that since the ease of discharge of hydroxide ions is more than bromide ions therefore, hydroxide ions will move towards the anode.
However, if we have concentrated aqueous potassium bromide, the bromide ions will move towards the anode instead of hydroxide ions because of their (greater) concentration.
Note that concentration has no effect on the positive ions (cations).
In the example of concentrated aqueous copper chloride (CuCl2), we have copper ions (Cu2+), chloride ions (Cl-), hydrogen ions (H+) and hydroxide ions (OH-).
In this case, the copper ions will move towards the cathode because they have greater ease of discharge. Thus, copper metal will be deposited at the negative electrode.
However, chloride ions will move towards the anode (instead of hydroxide ions) because they are greater in concentration. As a result, chlorine gas will be evolved at the anode (indicated by bubbles on the surface).

Question: What happens when concentrated aqueous potassium iodide is electrolysed?
When it comes to the cations, a hydrogen ion will move towards the cathode to form hydrogen gas by gaining electrons (reduction).
However, iodide ions are greater in number than hydroxide ions (even though the ease of discharge of hydroxide ions is greater). As a result, iodide ions will move towards the anode to be oxidised
This explains that the violet colour at the anode is due to iodine. This takes us to another very important topic which is about the uses of this method.
Uses of electrolysis:
Extraction of aluminium:
We use electricity to extract aluminium metal from its ores which make this metal very expensive as a large amount of electricity is required for this process.
Bauxite (sedimentary rock with high aluminium content), an aluminium ore, is used for this process.
- The free-moving ions in aluminium oxide ensure that the electricity is conducted during the process. We dissolve aluminium oxide in molten cryolite for some reasons.
Firstly, the melting point of aluminium oxide is more than 2000°C which means that a lot of heat energy is required to melt this compound. This can be very expensive.
Secondly, aluminium oxide does not dissolve in water. However, it dissolves in molten cryolite.
- This reduces the melting point of aluminium oxide so that less heat energy is required (comparatively cost-effective).
- The electrodes during this process are made up of graphite which is continuously replaced because carbon reacts with oxygen to form carbon dioxide which bubbles off.
- The positive aluminium ions gain electrons at the cathode to form molten aluminium while the oxide ions get oxidised (lose electrons) to form oxygen molecules.
The reaction at the cathode is:
Al3+ + 3e- → Al
This method shows how aluminium is extracted. This metal is soft and malleable and therefore, it is used to make cooking utensils, window frames, foils and even parts of the aeroplane.
Similarly, it is also used in spacecraft components, industrial appliances, power lines, ships and consumer electronics due to its specific properties.
Purification of copper:
We need pure metals widely in our industries. But, the metals we extract from the ores are not completely pure. As a result, we use electrolytic purification to obtain pure metals.
For purification of copper, we will aqueous copper (||) sulphate (any copper solution can be used) and the anode (positive electrode) is made up of impure copper while the cathode is made from pure copper.
When an electric current is passed, the impure copper anode starts to dissolves and this is shown by a decrease in mass. The impurities settle at the bottom of the solution.
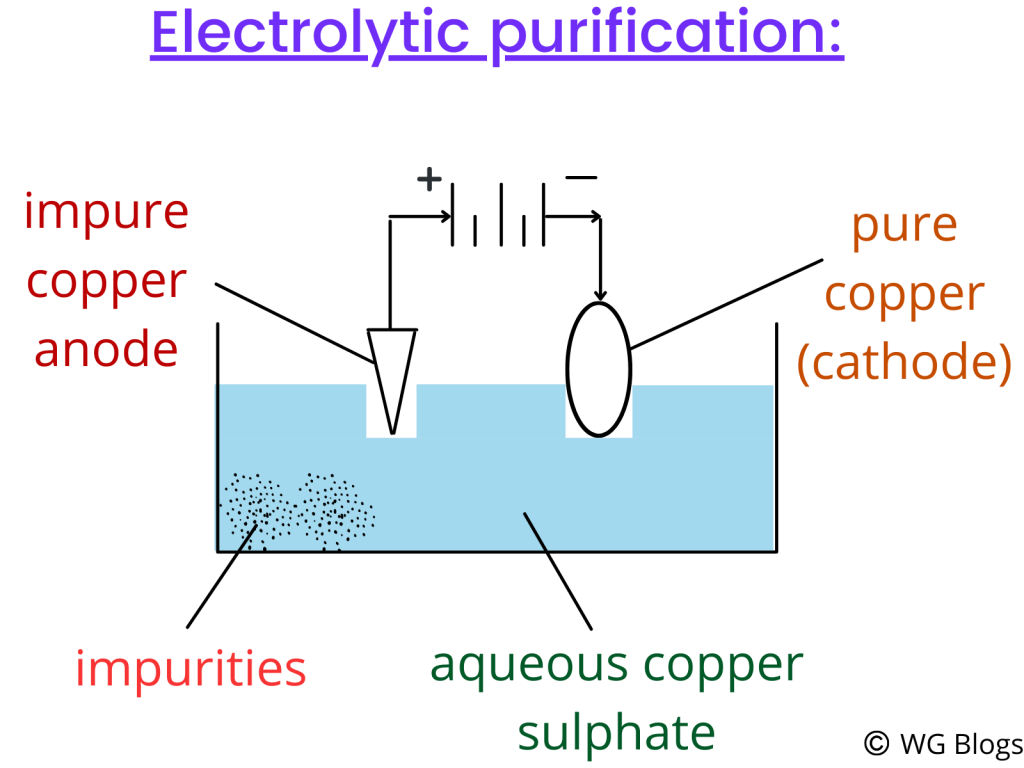
The mass of the cathode increases during the process. Do you know why?
This is because the copper from the anode is deposited at the cathode where copper ions are reduced to copper. Look at the equations below for further explanation.
The equation at the anode is:
Cu → Cu2+ + 2e-
The equation at the cathode is:
Cu2+ + 2e- → Cu
This is how we can obtain pure copper for industrial processes.
Electroplating:
Electroplating refers to the processes that create a metal coating (on a solid substrate) as the cation of the metal reduces due to the electric current.
In simple words, electrolysis is the deposition of a metallic layer on another substance by means of an electric current.
In this process, both cathode and anode are immersed in an electrolytic bath that contains a solution of the metal to be electroplated. When the current is passed, the metal is deposited onto the cathode (negative electrode).
This explains why the object that needs to be electroplated is at the cathode. The most commonly used metals (for electroplating) are:
- Copper
- Nickel
- Zinc
- Gold
- Tin
- Brass
- Chromium
- Platinum
This process is used to give a decorative touch to the object and prevent it from rusting (the coated layer protects the layer below from rusting).
For instance, if we want to electroplate an iron key with silver, we would use the following process.
The key (object to be electroplated) will be made from the cathode (of the electrolytic cell). The anode will be a bar of silver metal and the solution will be of silver chloride.
Object (key in this case) → Cathode
When an electric current is passed, the silver ions from the anode move towards the cathode and as a result, a layer of silver is coated on the iron key.
Simple cell:
Ever seen a battery and wondered how it works?
A simple cell (also known as an electric cell) is a device through which chemical changes are used to produce electricity (due to chemical reactions).
If we talk about its composition, it is made by inserting two metal electrodes in an electrolyte (electrolytic bath).
The more reactive metal undergoes oxidation (loss of electrons) which will cause the less reactive metal to become the cathode (in this case, we will take the cathode as the “positive electrode” although it is generally negative).
Due to this, a voltage (potential difference) will be generated between the metal electrodes. The greater apart the two metals are in the reactivity series, the greater will be the potential difference (voltage).
For example, if we take magnesium and zinc as the metals, the voltage produced will be approximately 1.1V. However, the combination of calcium and silver will give a voltage greater than 1.1V (because of the greater difference in the reactivity series).
Conclusion:
With this, the article has come to an end. I tried to cover all the important topics from the examination point of view.
The topics covered are electrolysis of molten, aqueous and concentrated compounds along with the uses of this process at the concept of simple cells.
You should practise a variety of past paper questions to ace this topic. Thank you very much for reading and staying with me till the end. Stay for more.

Thanks really helped
Glad about that!