An oxidation-reduction reaction (redox reaction) is a type of chemical reaction that involves the movement (transfer) of electrons.
In other words, the reactions that involve oxidation and reduction simultaneously refer to redox reactions. Similarly, the oxidation states (degree of oxidation for an atom) of an atom also change.
This was all about the introduction of this topic. Now, let’s discuss some important concepts in detail without further introductions.
Redox reactions
When is a substance oxidised?
Oxidation takes place if a substance:
- Loses electrons
- Loses hydrogen
- Gains oxygen
- Increase in oxidation state after the reaction
What do we mean when we say that oxidation occurs when a substance gains oxygen? Let me explain this with the help of an example.
2Mg + O2 → 2MgO
The above reaction shows magnesium burning in oxygen. In other words, magnesium has gained oxygen and it has been oxidised to magnesium oxide.
Similarly, what do we mean by a substance losing hydrogen?
CH4 + 2Cl2 → C + 4HCl
In the reaction above, methane (CH4) has been oxidised because it has lost hydrogen and the loss of hydrogen is oxidation.
Likewise, when hydrogen sulphide gas is mixed with chlorine gas, hydrogen chloride (a choking gas) is produced along with sulfur. The equation below represents the above information.
H2S + Cl2 → S + 2HCl
When is a substance reduced?
In simple words, the reduction reaction is completely opposite to oxidation reaction. In reduction, the substance:
- Gains electrons
- Gains hydrogen
- Loses oxygen
- A decrease in oxidation state after the reaction
What do we mean when we say that reduction is when a substance loses oxygen? Let’s take a look at an example to understand that.
2PbO2 → 2PbO + O2
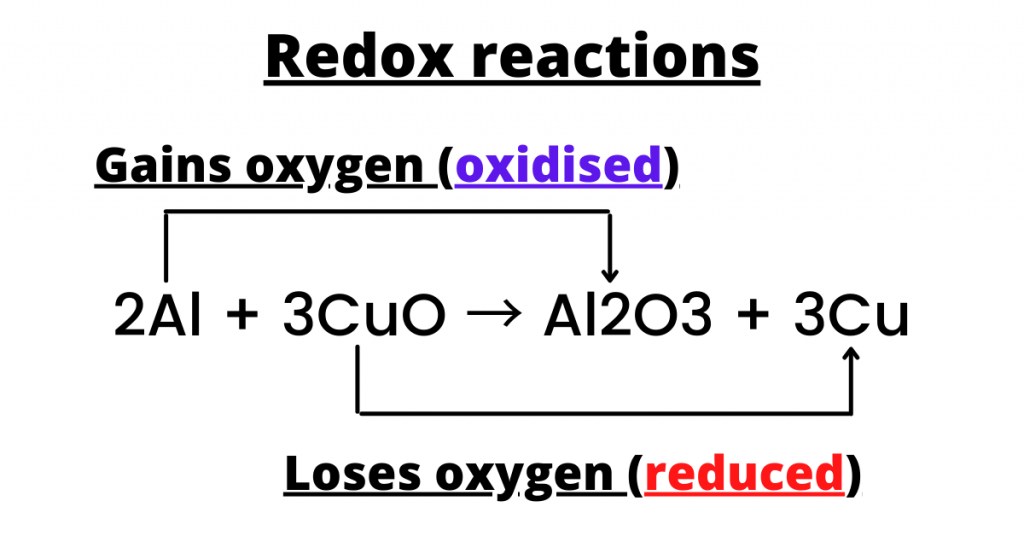
In the example above, lead dioxide is clearly being reduced because it is losing oxygen while oxygen itself is being oxidised. Apart, when copper (||) oxide and aluminium are heated, they react to form copper and aluminium oxide.
Thus, copper is being reduced because it is losing oxygen.
When a substance gains hydrogen, we say that it is reduced. For instance, when methane (CH4) reacts with chlorine, carbon and hydrogen chloride is formed.
We say that chlorine is reduced because it gains hydrogen.
A redox (derived from reduction and oxidation) reaction is a reaction in which both oxidation and reduction occur. For example, the reaction between aluminium and copper oxide is a redox reaction.
This takes us to another very important concept in this topic which is about oxidation state (increase or decrease) and loss or gain of electrons. So, let’s now understand that topic as well in detail now.
Oxidation and reduction (electron transfer):
Oxidation occurs when a substance loses electrons. The reaction below shows the oxidation of magnesium (since it is losing electrons).
Cu2+ + Mg → Cu + Mg2+
In the reaction, magnesium has lost an electron to become magnesium ion (it has been oxidised). However, the copper ion has gained electrons to become copper.
Further reading:
In other words, copper is reduced because it has gained electrons during the reaction. In order to learn this concept about electron transfer, you can use to the following acronym:
OIL RIG
Oxidation is loss (of electrons) – OIL
Reduction is gain (of electrons) – RIG
Now, let’s take a look at another example of electron transfer and study the reaction between copper and silver nitrate solution.
In the reaction, the copper atom loses electrons to become copper ion (thus it has been oxidised) while the silver ion has gained an electron to become silver (solid).
Therefore, it can be said that silver has been reduced because it has gained an electron.
This takes us to another very important topic which is about the oxidation state. So, let’s dive into the topic.
Oxidation state:
The oxidation state (sometimes referred to as oxidation number) is the degree of oxidation for an atom in a chemical compound. It is the charge that the atom of an element would possess as an ion.
Note: The oxidation state of elements is zero (0).
The oxidation states of some atoms (in a substance) are shown below:
- Li+ (oxidation state = +1)
- Na+ (oxidation state = +1)
- O2- (oxidation state = -2)
- Some transition metals have variable oxidation states such as Fe2+ and Fe3+
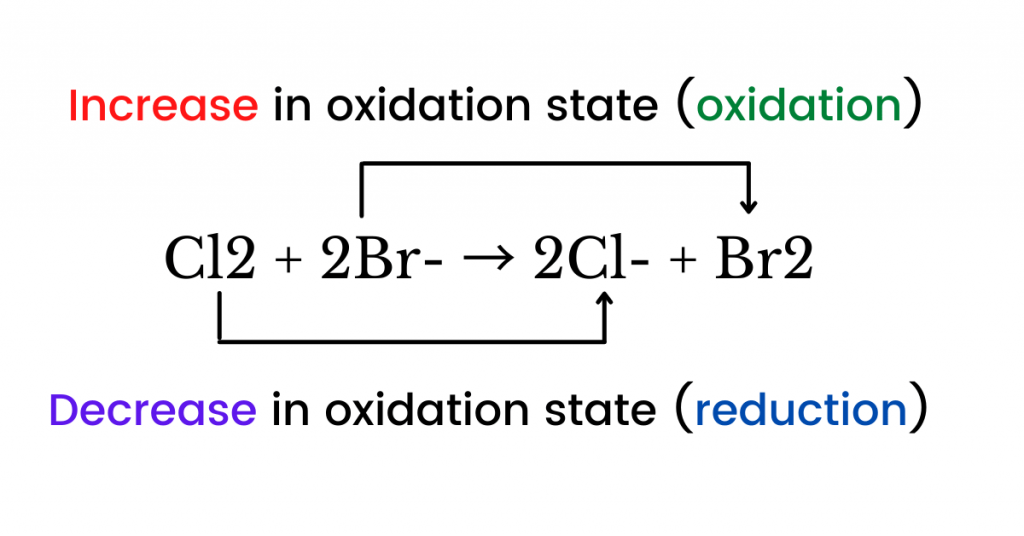
We discussed that oxidation occurs when an atom loses electrons which means that its oxidation state increases. When an atom gains electrons, the oxidation state is decreased and thus, the atom is reduced.
The equation below shows the oxidation of bromide ion and the reduction of chlorine molecules.
Note that not all chemical reactions are redox (oxidation-reduction) reactions because there are non-redox reactions such as precipitation and neutralization reactions.
The reaction between hydrochloric acid (HCl) and sodium hydroxide (NaOH) is an example of non-redox reaction (because it is a neutralization reaction).
Oxidising and reducing agents:
An oxidising agent is a substance that itself get reduced in the process to cause another substance to be oxidised. An oxidising agent:
- Is normally a positive ion or a non-metal
- Gains electrons from other ions or atoms to get reduced
- Causes oxidation reactions to occur
Oxidising agents can be used to kill inactive viruses, fungi and bacteria and they are used in a chemistry lab for the formation of alcohols and carboxylic acids.
Oxidising agents can also be used to break down coloured compounds (for example in clothes and hairs).
However, a reducing agent is a substance that itself get oxidised to cause another substance to get reduced. A reducing agent:
- Is normally a negative ion or a metal
- Loses electrons to other ions or elements
- Itself get oxidised
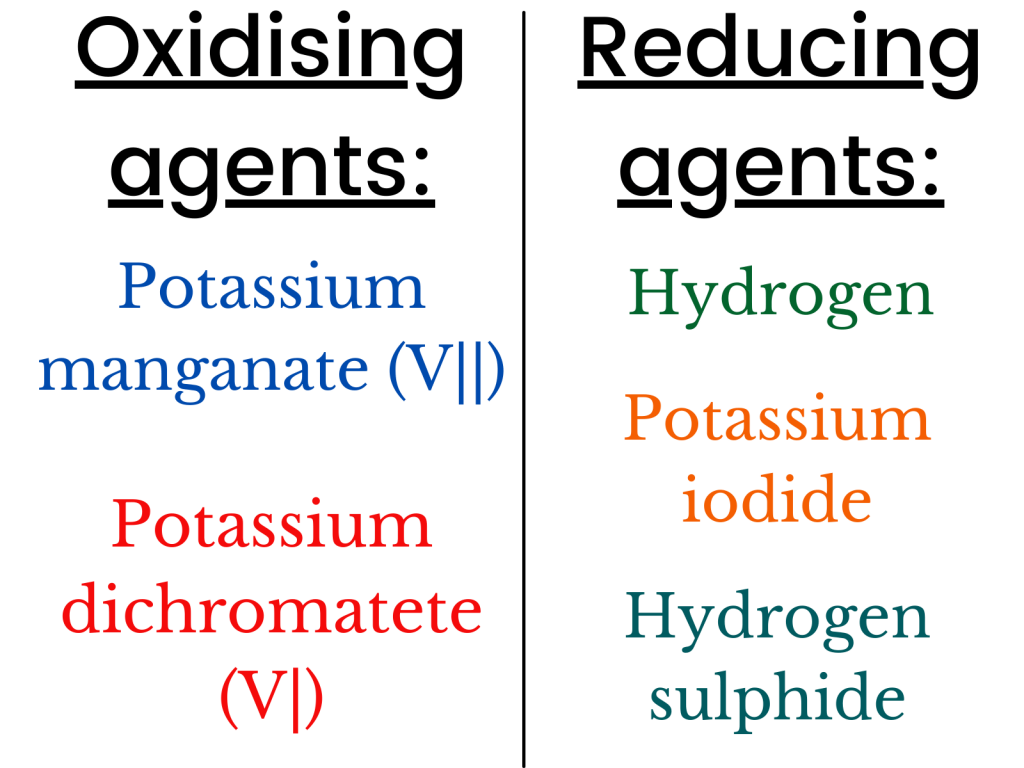
The table below shows some of the oxidising and reducing agents.
Moreover, you should also know how can we identify oxidising and reducing agents through tests.
In order to test for an oxidising agent, you can use starch-iodide paper or aqueous potassium iodide. In the presence of an oxidising agent, the starch-iodide paper will turn from white to blue.
More GCE O Level resources:
Electrolysis Made Simple | GCE O Level Chemistry
If you add potassium iodide to an unknown solution, a brown solution will indicate the presence of an oxidising agent.
Test for a reducing agent:
- In order to test for solutions, add acidified potassium manganate (V||) to the unknown solution.
If the colour changes from purple to colourless, the test for a reducing agent will be positive.

- In order to test for gases, soak a filter paper with acidified potassium manganate (V||), KMnO4, at the mouth of the test tube.
The colour will change from purple to colourless if a reducing agent is present. These are the tests in order to identify oxidising and reducing agents. Now, below are some questions for you to practice in order to ace this topic.
Question: Identify if the following reactions are redox reactions or non-redox reaction:
- N2 + O2 → 2NO
Answer: Redox reaction because nitrogen is oxidised (oxidation state has increased) and oxygen is reduced.
- Zn + CuO → ZnO + Cu
Answer: Redox reaction because zinc is oxidised and copper ions are reduced.
- Na2SO4 + BaCl2 → 2NaCl + BaSO4
Answer: It is a non-redox reaction because it is a precipitation reaction (two soluble compounds are reacting to form an insoluble compound).
Conclusion:
With this, the topic of redox reactions has come to an end. Thank you very much for reading and staying with me till the end. You should practice plenty of past paper questions to ace this topic.
Some of the topics covered in this article are listed below for your reference.
- What is meant by redox reactions?
- What is oxidation?
- What is meant by reduction?
- How to identify redox reactions
- What are oxidising and reducing agents?
Stay tuned for more.
