You will love this comprehensive guide if you want to learn about hydroxy compounds.
In fact, this is the exact resource I used to ace organic chemistry.
But first of all, let’s start with a simple introduction.
Hydroxyl or hydroxy is a functional group with the chemical formula -OH. For example, alcohols have the hydroxy functional group (-OH).
To be precise, we will look at alcohol and phenol in this article.
Hydroxy Compounds:
Alcohols:
Before talking about this topic, let’s recap what we learned about acyl chlorides.
Acyl chlorides (acid chlorides) are reactive derivatives of carboxylic acids. This simply means that they are derived (formed) from carboxylic acids.
You should know that acyl chlorides are represented as:
R-COCl
Here, R is a side chain.
Now, let’s study the reaction of acyl chlorides with alcohols.
Reaction of Acyl Chlorides with Alcohols:
I have a question for you.
What makes acyl chlorides reactive? Here’s the concept you need to know.
Recall the general formula of acyl chlorides: R-COCl
The carbon atom is bonded to highly electronegative chlorine and oxygen atoms. This makes the carbon atom highly positive. As a result, it attracts nucleophiles strongly.
Note: This is because nucleophiles are negatively charged as they contain a lone pair of electrons. In simple words, they are electron-rich species. Therefore, they are attracted to the positive carbon atom.
Now, let’s take a look at the concept.
Concept 1:
When alcohols react with acyl chlorides, esters are formed.
This reaction is vigorous and white fumes of HCl gas are formed. Here is an example:
Ethanol + Ethanoyl Chloride → Ethyl Ethanoate + Hydrogen Chloride
In this reaction, ethanoyl chloride reacts instantly with cold ethanol to give hydrogen chloride (steamy acidic gas).
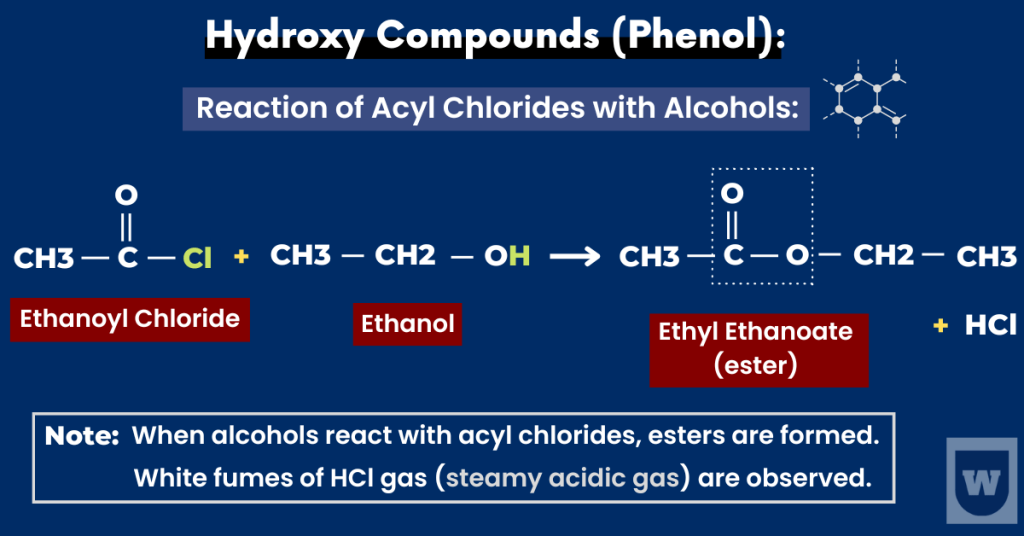
Remember that this is a nucleophilic substitution reaction. This takes us to the next concept.
Reaction of Acyl Chlorides with Phenols:
First of all, let’s talk about phenols.
Phenol is an aromatic alcohol. In simple words, phenols are organic compounds that have a hydroxyl group (OH) attached directly to a benzene ring.
We will take a look at phenols in detail. But for now, here’s what you need to know.
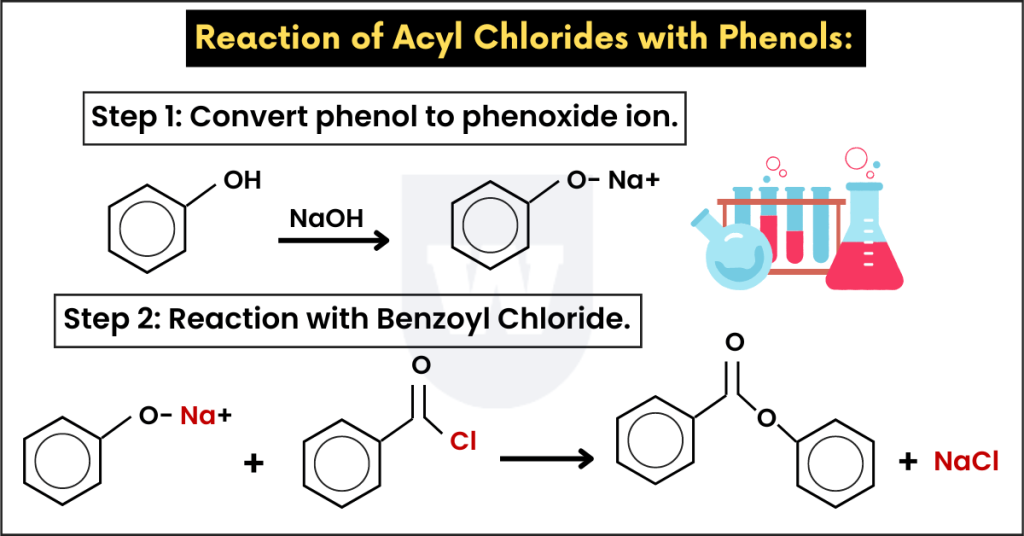
Acyl chlorides react with phenols to form esters. Pretty similar to the previous reaction, isn’t it?
Unlike the reaction with alcohol, this reaction requires a base. This base converts phenol to a phenoxide ion.
Now the question is, why do we need this phenoxide ion instead of phenol. Remember that the phenoxide ion is a better nucleophile. So, the reaction occurs more rapidly.
Here’s an example.
Benzoyl Chloride + Sodium Phenoxide → Phenyl Benzoate + NaCl
For this reaction, phenol was dissolved in the sodium hydroxide solution to get sodium phenoxide.
It reacted with benzoyl chloride (acyl chloride) to form an ester (phenyl benzoate).
With this, let’s move on straight to the next topic.
Phenol:
Production of phenol:
Before talking about the reactions, let’s see how phenol is formed.
Here’s a step-by-step guide.
Step 1:
First of all, we need nitrous acid (HNO2).
For that, sodium nitrate will react with hydrochloric acid. Here’s the reaction:
NaNO3 + HCl → HNO2 + NaCl
When you have nitrous acid, move on to the next step.
Step 2:
The nitrous acid reacts with Phenylamine to form unstable diazonium salt.
Note: The positive ion containing the -N2 + group is called the diazonium ion. We will take a look at it later in this article.
As the diazonium salt is unstable (highly reactive), warming it with water leads to the formation of phenols.
Now that you know about the production of phenols, let’s take a look at their reactions.
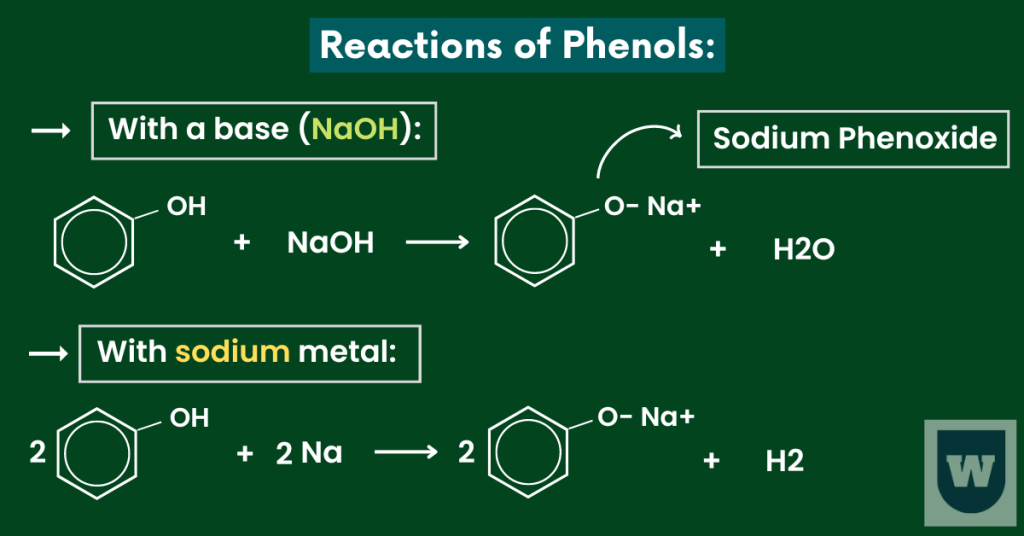
Reaction of phenols with bases:
The concept is simple.
As phenol is acidic in nature, it reacts with bases to form salt and water. Here’s an example.
Phenol + Sodium Hydroxide → Sodium Phenoxide + Water
Note that the salt (sodium phenoxide) is soluble in water.
(Take a look at the image below to understand this).
Reaction of phenol with sodium metal:
When phenol reacts with sodium metal, it forms sodium phenoxide (salt) and hydrogen gas.
Important Note: To make things easier, take phenol as an acid. Acids react with bases to form salt and water.
This is the case with phenol as well. Now, let’s move on to the next reaction.
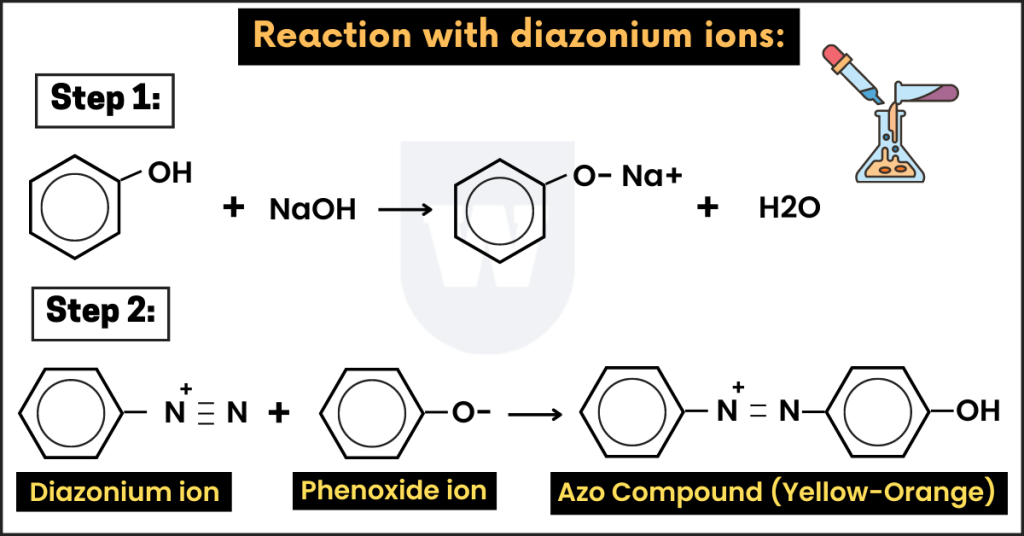
Reaction with diazonium ions:
Before talking about this reaction, note that diazonium ions are reactive compounds.
They contain an -N2+ group. Let me explain this to you.
- First of all, we dissolve phenol in a sodium hydroxide solution to get sodium phenoxide.
Now, this solution is cooled in ice.
- The sodium phenoxide we prepared above reacts with diazonium ion to form a yellow-orange precipitate.
We call them azo compounds. Azo compounds contain two benzene rings linked by a nitrogen bridge.
With that being said, let’s move on to the next reaction.
Before studying the next reaction, let’s study the 2,4,6 positions.
2,4,6 Directing Groups:
Here’s the concept.
We have a hydroxyl group (OH) attached to a benzene ring.
The lone pair on the oxygen atom overlaps with the pi system of the benzene ring. Now you might be wondering, why is this important?
Look, this increases the electron density in the aromatic ring. So, the incoming electrophiles (such as NO2+) are directed to the 2, 4 and 6 positions.
Let me make things simpler for you.
Let’s say we have phenol and an electrophile (NO2+) attacks it. This electrophile will be directed at positions 2 and 4.
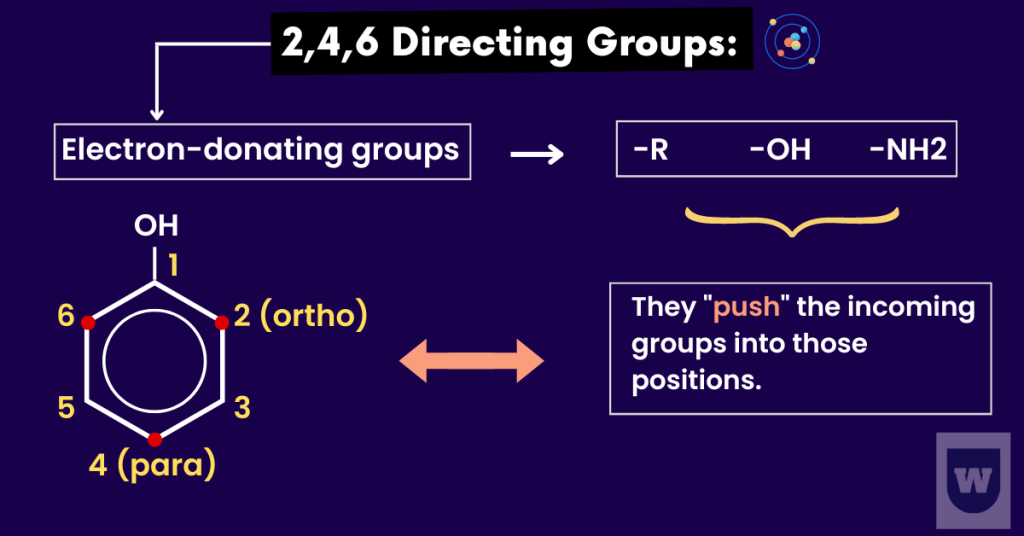
Here’s the summary:
Due to a lone pair on the oxygen atom:
- The electron density is greatest at the 2, 4 and 6 positions.
- So, the substitution takes place at the 2, 4 and 6 positions.
The table below shows this:
| Groups that direct the incoming electrophile to the 2- or 4- positions | Groups that direct the incoming electrophile to the 3- position |
|---|---|
| –NH2, –NHR or –NR2 | –NO2 |
| –CH3, –alkyl | -CN |
| –OH or –OR | –NH3 |
| –NHCOR | –CHO, –COR |
| –Cl | –CO2H, –CO2R |
Note: Instead of 2 and 4 positions, some groups (such as CN) direct the electrophile to the 3 or 5 positions.
Simple, isn’t it?
Let’s take a look at some examples to better understand this.
Nitration of phenols:
(Using Diluted HNO3):
In this reaction, phenol is nitrated with dilute nitric acid.
Due to the hydroxyl group, the electrophile (NO2+) is directed to the ortho para (2 and 4) direction. In simple words, this reaction gives us a mixture of ortho and para nitrophenols.
Now you might be wondering, what will happen if we use concentrated nitric acid.
(Using Concentrated HNO3):
When we treat phenol with concentrated nitric acid, the nitration leads to the formation of 2, 4, 6-trinitrophenol.
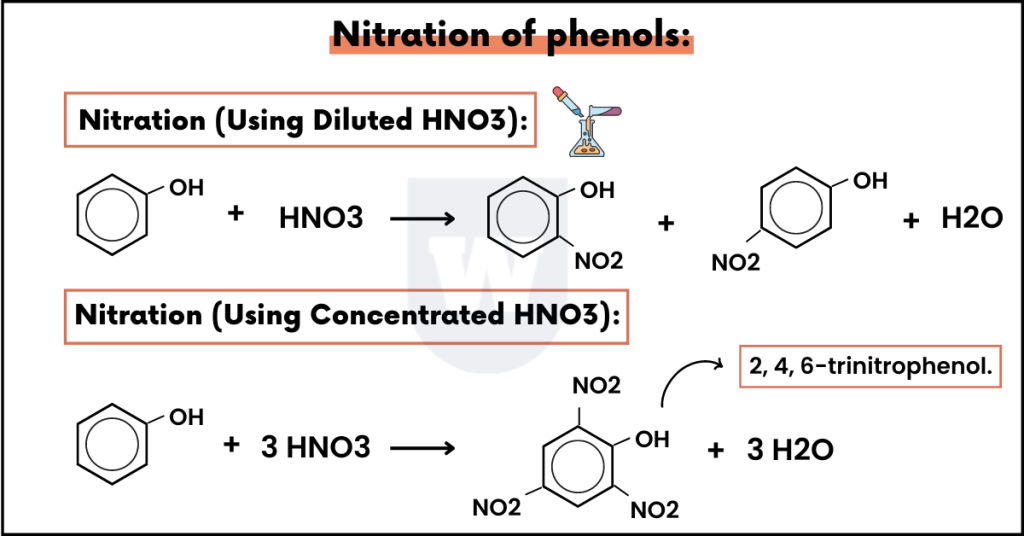
Fun Fact: It is also known as picric acid and is highly acidic in nature.
Now, let’s move on to the next reaction.
Bromination of phenols:
This concept is extremely important.
Remember that phenols react rapidly with bromine water without a catalyst.
In this reaction, the orange solution is decolourised and a white precipitate of 2, 4, 6-tribromophenol is formed.
We use this reaction as a test for phenols.
With this, let’s move on to the next topic.
The acidity of phenol:
As we discussed earlier, phenol is acidic in nature. But, why is this so?
A hydrogen ion breaks from the -OH group attached to the benzene ring.
As phenol is a proton (H+) donor, in this case, it behaves as an acid. Note that when this hydrogen ion is lost, a phenoxide ion is formed.
This means that we have O- attached to the benzene ring instead of OH.
Now, here’s something interesting.
The lone pair on the oxygen atom overlaps with the delocalised electrons in the benzene ring. This makes the phenoxide ion more stable as the negative charge now spreads throughout the ion.
Note: As the oxygen atom is the most electronegative element in the phenoxide ion, it will attract the hydrogen ion back again.
This is the reason phenol is a weak acid.
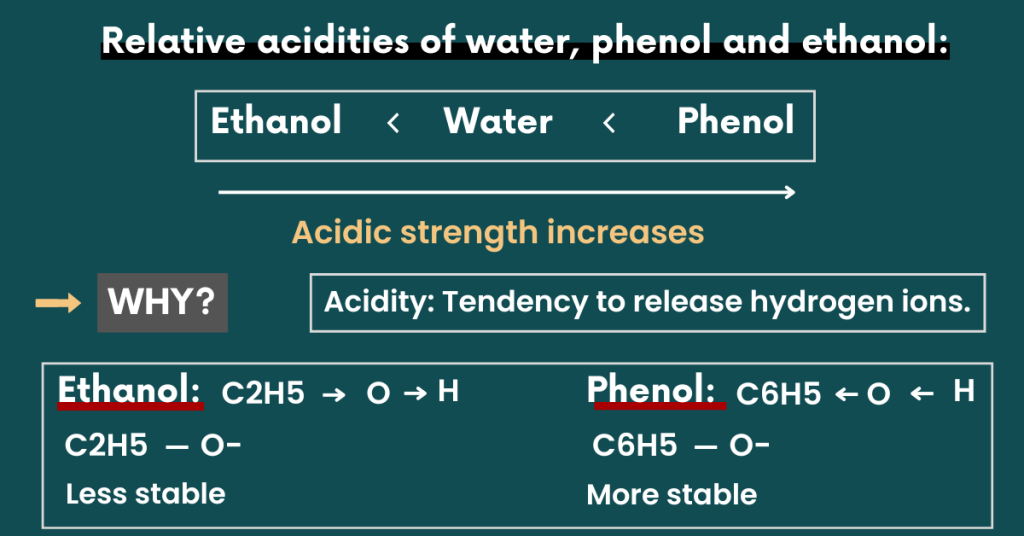
Relative acidities of water, phenol and ethanol:
Ethanol is the weakest acid while phenol has the greatest acidic strength.
Here’s why.
Recall that the strongest acid will most rapidly ionize (release H+ ions). Although the OH bond in ethanol is polar, its polarity is reduced by the electron-donating effect of the ethyl group.
In other words, it pushes the electrons towards the hydrogen atom. This reduces the ability of hydrogen to ionize.
Now the question is, why is phenol the strongest acid?
Recall that phenol is aromatic alcohol in which an OH group is directly bonded to a benzene ring. The OH bond in phenol is also polar like ethanol.
But, its polarity is increased by the electron-withdrawing effect of the phenyl group.
Let me explain.
The phenyl group pulls the electrons towards itself. This increases the partial positive charge of hydrogen allowing it to ionize quickly. This contributes to the acidic strength of phenol.
Simple, isn’t it?
Wrapping Up:
With this, our topic about hydroxy compounds (phenol) has come to an end.
To recap, we studied the production and reactions of phenol. If you want to ace this topic in your exam, do practise past paper questions.
Thank you for reading and staying with me till the end. Stay tuned for more.
Further reading:
Hydrocarbons (Arenes) | A2 Organic Chemistry Notes
Carboxylic Acids and Derivatives | A2 Organic Chemistry (9701)
