If you want to learn about the atomic structure for Oxygen (O2), you will love this comprehensive guide. But first of all, what is meant by the atomic structure?
Let me tell you.
We know that an atom consists of a positively charged nucleus. This nucleus contains protons and neutrons. This is followed by electrons (equal to the number of protons).
This concept refers to the atomic structure. Pretty simple, isn’t it?
In simple words, atomic models help us visualize (imagine) the internal structure of an atom.
Now, let’s take a look at some examples to further understand this topic.
Atomic structure for Oxygen (O2):
Before diving into more details, here are some concepts that you need to know.
The nucleus contains the majority of the atom’s mass. And it is surrounded by electrons in defined shells.
Note that the arrangement of these basic particles (protons, neutrons and electrons) results in the different properties of atoms.
Now, let’s take a look at oxygen (O2).
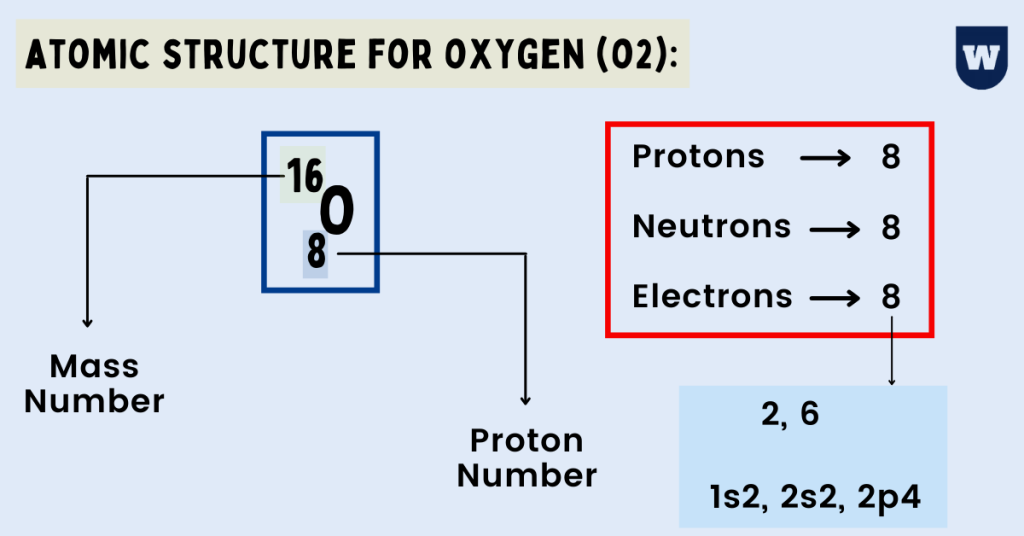
An Oxygen atom has:
- 8 protons
- 8 electrons
- 8 neutrons
To know more about the atomic structure of oxygen, you need to learn about the electronic configuration.
The electronic configuration shows the distribution of electrons in an atom. And, it can be shown in two ways:
- In the form of shells
- In the form of orbitals
Let’s talk about orbitals first.
In simple words, orbitals are a representation of the nature of electrons. We have 4 different types of orbitals:
s, p, d and f
Let’s talk briefly about s and p orbital because they are the most common in organic chemistry.
s orbital: They are spherical in shape and have a nucleus at their centre. Note that one “s” orbital can hold a maximum of 2 electrons.
p orbital: The p orbital has a dumbbell (lobed) shape. A single “p” orbital can hold a maximum of 6 electrons.
So, the electronic configuration of an Oxygen atom (in terms of orbitals) is 1s22s22p4.
There are more details about this later in this article. Keep reading.
As mentioned above, the second way of showing the electronic configuration is using shells.
An electron shell is an outside part around the nucleus of an atom. You can also say that a shell is a group of atomic orbitals. The closest shell to the nucleus is called the “1 shell”.
This is followed by the “2 shell”, “3 shell” and so on.
Now, here is the MAIN point. We can use shells to make the atomic structure of an atom. For that, you should know the following:
“1 shell”: Can hold a maximum of 2 electrons
“2 shell”: Can hold a maximum of 8 electrons
“3 shell”: Can hold a maximum of 18 electrons
…
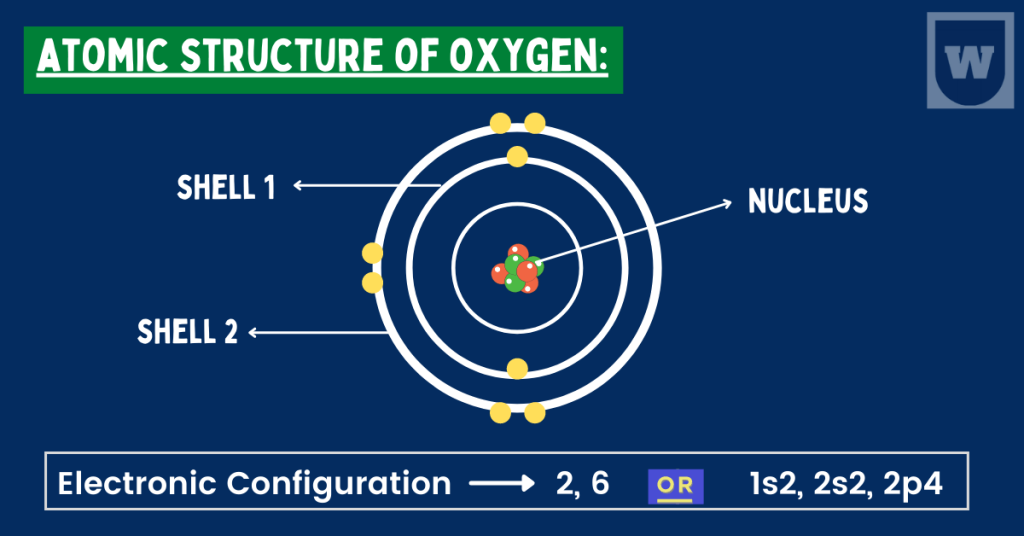
As you can see in the image below, an oxygen atom has 2 electrons in the first shell. And, it has 6 electrons in the second shell.
As a result, an oxygen atom needs two more electrons to become stable (according to the octet rule).
Summary: An Oxygen atom has 8 protons and electrons. Similarly, it has 8 neutrons as well. So diagrammatically, its atomic structure will have 8 electrons (2 in the first shell and 6 in the second shell) placed on the electrons shells (rings).
Take a look at the diagram again. You will notice that we have a nucleus at the centre of the atom. This nucleus is composed of protons and neutrons.
In simple words, this is the atomic structure for oxygen (O2).
Subatomic particles:
In Chemistry, we have three subatomic particles:
- Protons
- Neutrons
- Electrons
And, it is important to know about them to learn about the atomic structure of ANY element.
Further reading:
Covalent Bonding of Water | H2O | Best Guide
Ionic Bonding in NaCl (Sodium Chloride)
Proton, a subatomic particle, has a symbol of p and a positive charge of +1. Its mass is similar to that of a neutron (but is slightly less).
Moving on, let’s talk about neutrons.
Neutron is a neutral subatomic particle and has a symbol of n. Their mass is slightly greater than that of a proton. They, along with protons, form the nucleus of an atom.
Pretty simple, isn’t it?
Electron, represented by e, has a charge of -1, and its mass is also negligible.,
An atom’s electron configuration shows the position of the electrons in a typical atom. We can predict an atom’s properties, such as boiling point, using the electron configuration.
Atomic structure of isotopes:
First of all, let me introduce isotopes to you.
In simple words, isotopes are the atoms of the same element. They have the same number of protons and electrons (atomic number), but a different number of neutrons.
Here is an example.
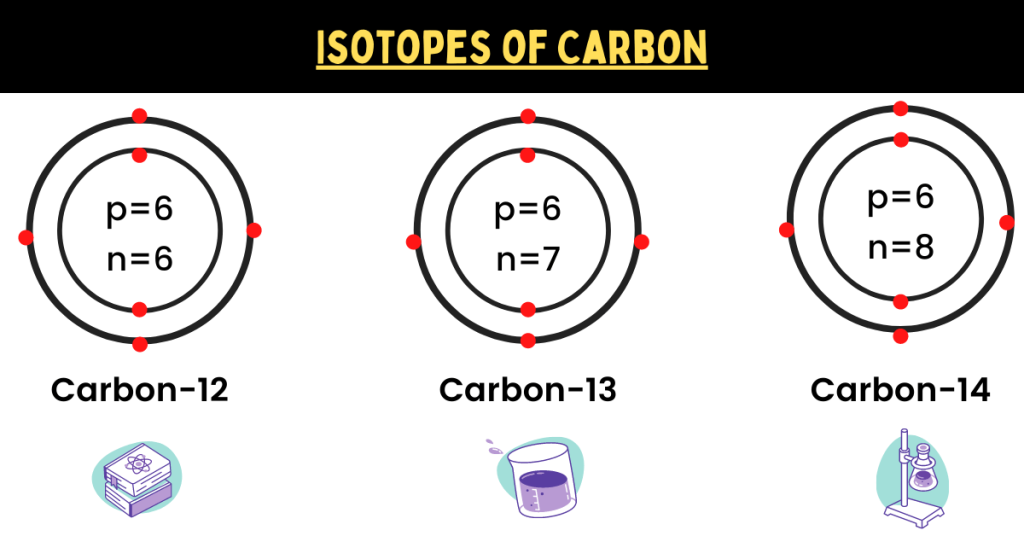
Carbon has an atomic number of 6 because it has six electrons. Now, we have three isotopes of carbon:
- Carbon 12 (containing 6 protons and 6 neutrons)
- Carbon 13 (containing 6 protons and 7 neutrons)
- Carbon 14 (containing 6 protons and 8 neutrons)
Note that this difference in the number of neutrons causes these isotopes to have different properties. In the diagram below, you can see the atomic structure of carbon (isotopes).
To further understand this topic, let me share some other examples for atomic structure.
Atomic structure of Sodium (Na):
Sodium (Na) is a soft silvery-white alkali metal and is present in group 1 of the periodic table.
Before talking about the atomic structure of sodium, let’s study its atom. A Sodium (11Na) atom has the following:
- Number of protons = 11
- Number of electrons = 11
- Number of neutrons = (23 – 11) = 12
Now, here is the electronic configuration of a neutral sodium atom (in form of shells): 2, 8, 1
So if you draw the atomic structure of Na, you will make 2 electrons in the first shell, 8 electrons in the second shell, and 1 electron in the third shell.
Now, what if I ask you to draw the atomic structure of a Sodium ion. How will you do so?
Here’s what you need to know. A Sodium ion (Na+) loses one electron to become stable. So, the electronic configuration will be:
2, 8
To draw the atomic structure, you will make 2 electrons in the first shell and 8 electrons in the second shell. Pretty simple, isn’t it?
Atomic structure of Helium (He):
Helium (represented by He) is an inert (unreactive) gas. Its atomic number is 2 and is the first in the noble gas group in the periodic table.
If we talk about its atomic structure, its nucleus contains two protons and two neutrons. This nucleus is encircled by two orbiting electrons.
In other words, we have two electrons in the first (outermost) shell of Helium.
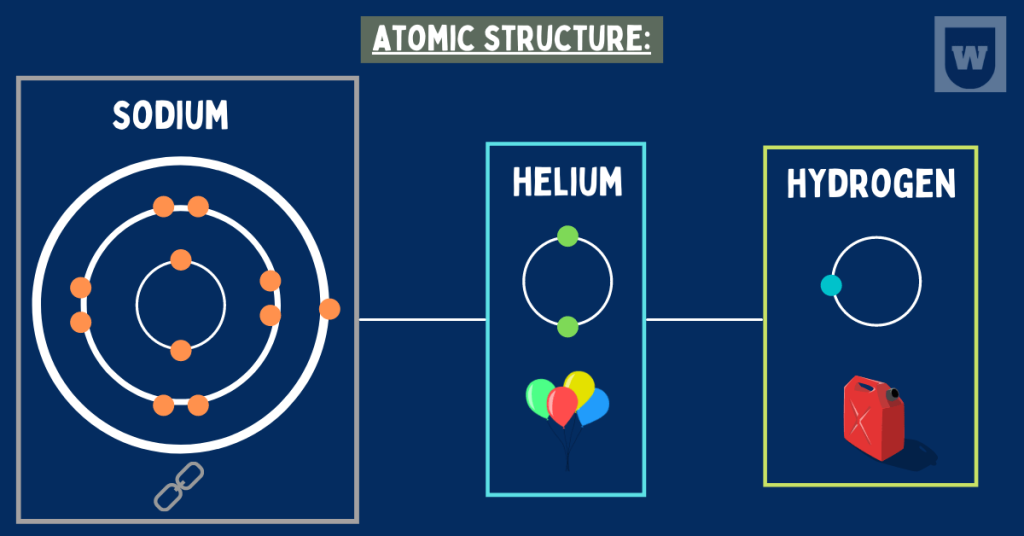
Talking about a Hydrogen atom, it has ONLY one electron in its first (outermost) shell. You can take a look at the above diagram for reference.
Atomic structure of Nitrogen (N):
Nitrogen, a chemical element with the symbol N, has an atomic number of 7. This means that Nitrogen has 7 protons and electrons.
Moving on to the electronic configuration of Nitrogen (N), its electronic shell structure is (2, 5).
This means that Nitrogen has 2 electrons in the first shell and 5 electrons in the second shell. The nucleus in the centre contains 7 protons and 7 neutrons (14 – 7) as well.
Wrapping up:
With this, our topic about the atomic structure for oxygen has come to an end. To recap, Oxygen has an atomic number of 8.
As a result, it has 2 electrons in the first and 6 electrons in the second shell.
Now, I turn it over to you. Which part of this topic do you find challenging? Is it drawing the atomic structure? Do let me know.
But before going, I have a question for you. Why is it important to know about the atomic structure of an atom? It gives us details about chemical reactions.
Do let me know about some other points in the comments below. Thank you for reading.
