You will love this in-depth guide if you want to learn about hydrocarbons (arenes).
Now the question is, what are arenes?
Arenes are organic compounds, but with a twist, they contain one or more benzene rings. Here’s what I mean.
Arenes are also known as aromatic or aryl compounds. The term “aromatic” simply shows that they have delocalised bonding.
I will explain this concept of delocalised bonding later in this article.
But for now, note that arenes are cyclic unsaturated compounds as they have a pi-bond system.
The structure of benzene:
Let’s look at benzene to better understand arenes (aromatic hydrocarbons).
Benzene is the simplest arene and has a molecular formula of C6H6. Here’s what you should know about the bonding in benzene.
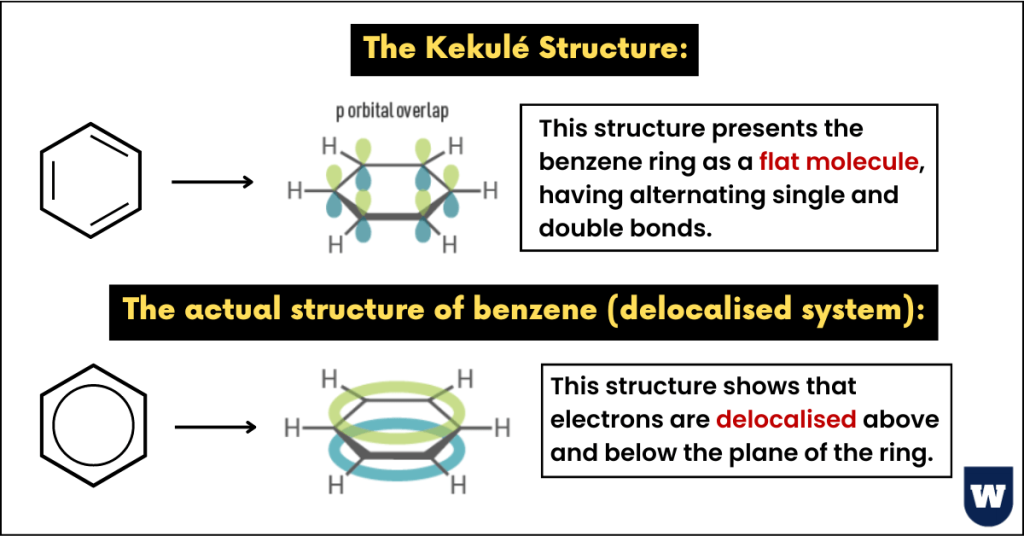
The Kekulé structure for benzene (C6H6):
Friedrich August Kekule, a German organic chemist, suggested a cyclic hexagonal structure of benzene.
He suggested that the carbon atoms in benzene are arranged in a hexagon, with alternating double and single bonds.
Plus, each carbon atom has hydrogen attached to it.
That’s it?
NO!
Now in science, we all know that older discoveries get replaced by newer ones. Guess what, evidence showed that the Kekulé structure was not correct.
Now you must be wondering, why is that so?
- Due to three double bonds, benzene was expected to react like alkenes.
First of all, let’s recall alkenes. They are unsaturated hydrocarbons containing a double carbon-carbon bond (C=C).
Recall that alkenes undergo addition reactions. However, benzene usually undergoes substitution reactions.
This information was enough to assume that there were no double bonds in benzene.
Further research showed benzene was a perfectly symmetrical (planar) molecule. Kekule’s structure suggested that benzene was non-planar.
Now, let’s talk about the actual structure of benzene.
The actual structure of benzene (delocalised system):
Here’s what you should know about the actual structure of benzene.
- Each carbon atom of benzene forms three sigma (σ) bonds.
We all know that carbon forms four bonds. So, now we have one electron spare on each carbon atom in its p orbital.
As there are six carbon atoms in benzene, therefore we have six free electrons in the p-orbitals.
Now that you know this, let’s talk about delocalised bonding
- These six electrons in the pi-bond are delocalised.
To be precise, delocalised electrons are not in any one particular position.
Instead, these electrons contribute to the pi-bond system that is spread over the whole structure.
So, here’s the summary:
- Benzene (C6H6) is a planar molecule.
- The six carbon atoms form a perfectly regular hexagon.
- All the carbon atoms in the benzene ring are sp2 hybridized (120° bond angle).
- There are delocalised electrons above and below the plane of the ring.
Now, let’s talk about the reactions of arenes.
Reactions of arenes (hydrocarbons):
Before talking about the reactions, here’s what you should know.
Benzene does not generally undergo addition reactions because arenes are stable compounds due to the delocalisation of pi (π) electrons.
As addition reactions involve the breaking up of the delocalised system, the majority of benzene’s reactions involve substitution.
We call this electrophilic substitution.
Substitution reaction:
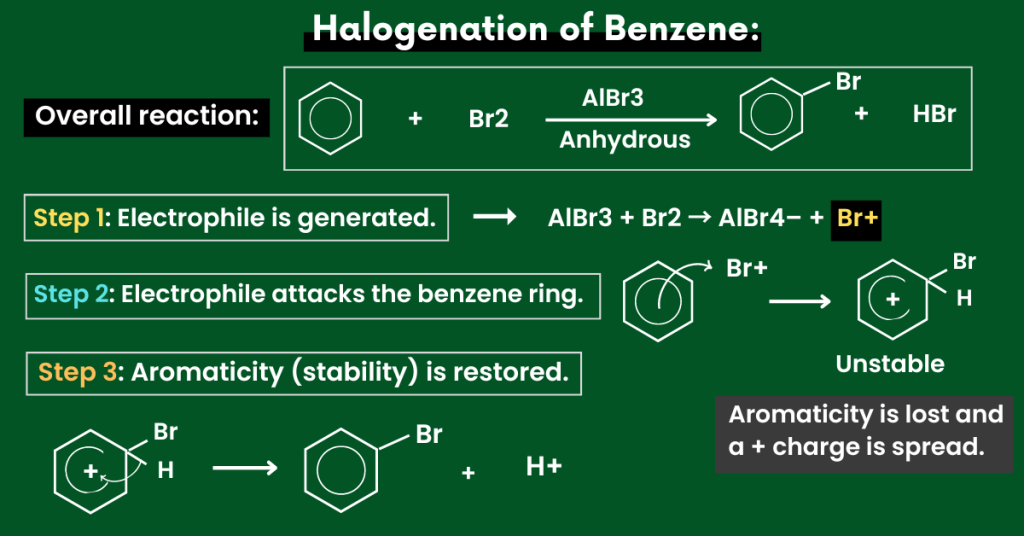
This reaction is also known as the halogenation of benzene.
This is because a halogen (chlorine or bromine) gets attached to the benzene ring. Here’s how:
(Taking the example of bromine).
- Reagent and condition: Bromine (Br2) and anhydrous AlBr3 as a catalyst.
- Electrophile: Br+
Now you might be wondering, how is this electrophile made? Here’s how:
AlBr3 + Br2 → AlBr4– + Br+
Now, this electrophile attacks the benzene ring and replaces the hydrogen atom.
In this reaction, the electrophile replaces a hydrogen atom on the benzene ring. Due to the stability of benzene, a catalyst (AlBr3) is also required.
Now, let’s take a look at the mechanism of electrophilic substitution.
Mechanism of electrophilic substitution:
Step 1:
- First of all, the electrophile is generated.
Note that electrophiles are electron-deficient species. So, they are attracted to an electron-rich centre.
As discussed above, the electrophile, in this case, is Br+.
When you have the electrophile, move on to the next step.
Step 2:
- Now, the electrophile carries out an electrophilic attack on the benzene ring.
The arrow from the benzene ring to the electrophile shows that a pair of electrons from the benzene ring is donated to the electrophile.
Due to this, the aromaticity (stability) in the benzene ring is disturbed.
This is why we have a positive charge inside the benzene ring.
Step 3:
- To restore the aromaticity (stability), the bond electrons from the C-H bond go into the benzene ring.
As a result, hydrogen is removed and the electrophile gets attached to the benzene ring.
Pretty simple, isn’t it? This takes us straight to the next reaction.
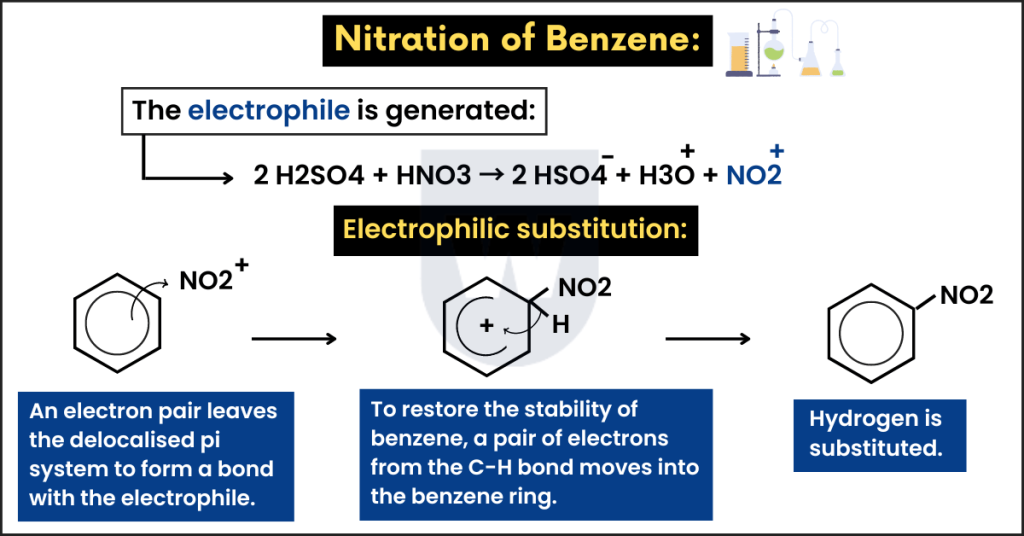
Nitration of benzene:
This reaction is similar to the previous one. However, the electrophile in this reaction is NO2+.
In this reaction, benzene is converted to nitrobenzene.
- Reagents: Concentrated nitric acid and concentrated sulfuric acid (catalyst).
- Condition: Heat under reflux at 55°C.
- Electrophile: NO2+ (nitronium ion).
Let’s see how this reaction occurs.
Step 1:
As we discussed earlier, the electrophile is generated. The reaction below shows this:
2 H2SO4 + HNO3 → 2 HSO4– + H3O+ + NO2+
Now, it is time to move on to the next step.
Step 2:
This electrophile (NO2+) attacks the benzene ring. So, an electron pair leaves the delocalised pi system to form a bond with the electrophile.
Remember that electrons move from the benzene ring to the electrophiles as they are electron-deficient species.
This disturbs the stability of benzene. As a result, an unstable intermediate is formed.
Step 3:
To restore the stability of benzene, a pair of electrons from the C-H bond moves into the benzene ring.
In this way, hydrogen is substituted. This is how nitrobenzene is formed.
With this, let’s move on to the next reaction.
Friedel–Crafts alkylation:
This is also an electrophilic substitution reaction. As we studied in the previous reactions, hydrogen is replaced.
In this reaction, hydrogen is substituted by alkyl groups such as CH3, C2H5 and C3H7.
Here’s what you need to know.
- Reagents: Haloalkane (RX) and anhydrous aluminium chloride (AlCl3).
You can use any chloroalkane. An example is CH3Cl.
- Conditions: Room temperature and a dry inert solvent (such as dry ether).
- Electrophile: A carbocation (R+). For example, if the reagent is CH3Cl, CH3+ will be the electrophile.
Here’s another example. If the chloroalkane (reagent) is C2H5Cl, C2H5+ will be the electrophile.
Let’s take a look at it in more detail.
Step 1:
First of all, the electrophile is generated. Let’s say that we have C2H5 (CH3CH2) as a reagent.
The equation below shows how the electrophile is generated:
CH3CH2Cl + AlCl3 → CH3CH2+ + AlCl4_
Note that CH3CH2+ is the electrophile. As we discussed earlier, an electrophilic substitution reaction occurs.
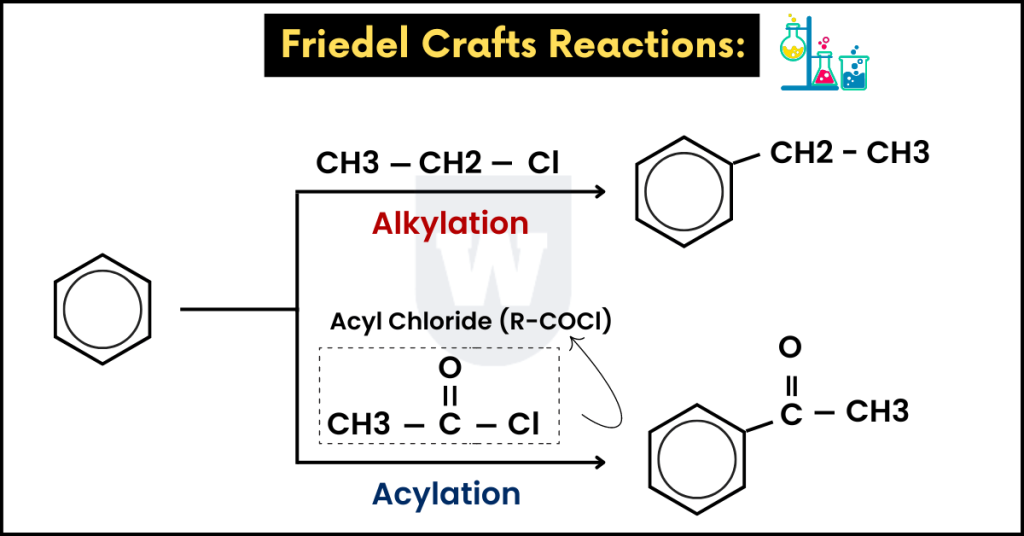
To be precise, CH3CH2 is attached to the benzene ring.
Important Note:
As AlCl3 is a catalyst, it has to be regenerated at the end of the reaction. The reaction below shows this:
AlCl4_ + H+ → AlCl3 + HCl
So, this is how the regeneration of the catalyst takes place. Note that this question is often asked in the exam.
Exam tip: Let’s say that you have to identify the reagent from the final product. For that, simply add a Cl to the electrophile attached to the benzene ring.
For example, if CH3CH2CH2 is attached to the benzene ring, CH3CH2CH2Cl is the reagent.
Now, let’s take a look at a similar reaction.
Friedel–Crafts acylation:
This reaction involves the substitution of acyl groups on the benzene ring.
Recall that acyl chlorides are represented by R-COCl (where R is an alkyl group).
So, here’s what you need to know:
- Reagents: An acyl chloride and anhydrous AlCl3 as a catalyst.
- Condition: Room temperature and a dry inert solvent (ether).
- Electrophile: RCO+
As we studied earlier, the electrophile is generated. It then attacks the benzene ring, forming an unstable intermediate.
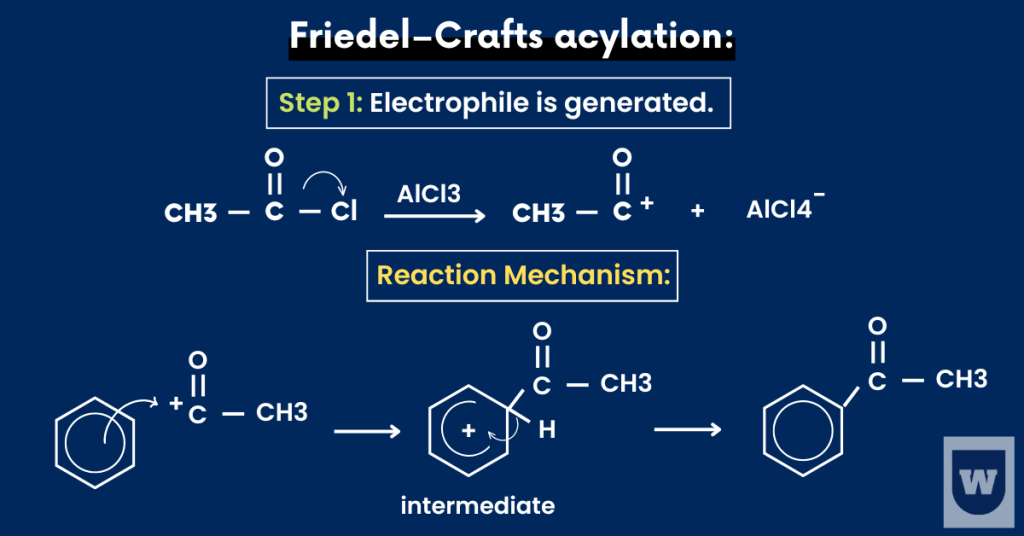
To regain its stability, benzene loses a hydrogen atom, and the electrophile is substituted in its place.
The role of a catalyst:
I have a question for you. Why do we need a catalyst in alkylation and acylation?
Here’s why.
The attacking species are not strong electrophiles. The catalyst makes the attacking species more positive.
As you already know, electrophiles are electron-deficient species. So, this allows them to attack the benzene ring more rapidly.
Simple, isn’t it?
Further reading:
Inorganic Chemistry | AS Level | In-depth Notes
Now, let’s move on to the next reaction.
Hydrogenation of benzene:
In this reaction, the reduction of benzene produces cyclohexane (C6H12).
In short, every carbon atom gains one hydrogen atom and becomes sp3 hybridized. The bond angle is now 109.5 degrees, and the shape is called tetrahedral.
Here are the reagents and conditions:
- Reagents: H2 gas
- Conditions: Nickel (Ni) metal as a catalyst (180 – 200°C).
Note that high temperature or pressure is necessary for this reaction to occur. This is because benzene is stable due to its delocalised pi-bond system.
Oxidation of alkyl side chain of benzene:
This reaction frequently appears in exams.
I have a question for you. What does the alkyl side chain of benzene mean?
This means that an alkyl group (such as CH3CH2) is attached to the benzene ring. This side chain is oxidised to a single carboxylic acid (-COOH) group.
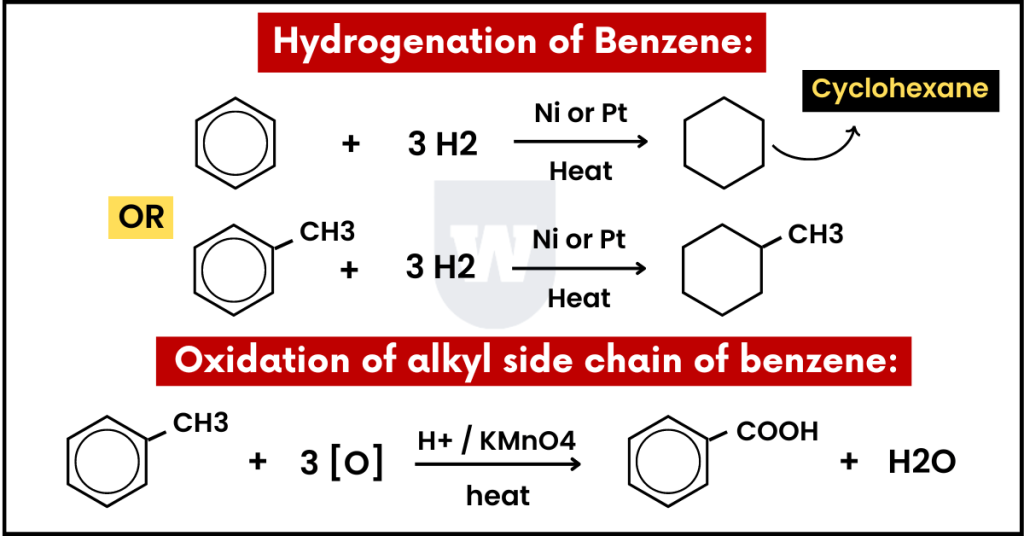
Here’s how:
- Reagents: Hot alkaline KMnO4 and then dilute acid.
- Conditions: Heat under reflux
This results in the formation of benzoic acid.
Note that the benzene ring is NOT oxidised. The alkyl side chain is oxidised to -COOH. With this, let’s move on to the next topic.
Methylbenzene (Toluene):
Note that methylbenzene is also known as toluene. This means that a methyl group (CH3) is attached to benzene.
The formula is C6H5CH3. Here’s what you need to know.
Halogenation:
Depending on the conditions, halogenation can occur:
- In the aromatic ring
- In the side chain
Let me explain.
(In the aromatic ring):
If we have bromine (Br2) and AlBr3 as a catalyst, electrophilic substitution will occur. Therefore, hydrogen will be replaced by bromine.
In simple words, the halogen (bromine) will attach to the benzene (aromatic) ring.
(In the side chain):
But if there is UV light, the alkyl side chain will undergo free-radical substitution. This simply means that hydrogen will be replaced by a halogen.
Pretty simple, isn’t it?
2,4,6 Directing Group:
In methylbenzene, the methyl group (CH3) is a 2,4,6 directing group.
Let me explain this to you.
The methyl group is said to be 2,4 directing as it pushes the incoming groups into those positions. Here is an example.
Let’s say we have methylbenzene and an electrophile (NO2+) attacks it. This electrophile will be directed at positions 2 and 4.
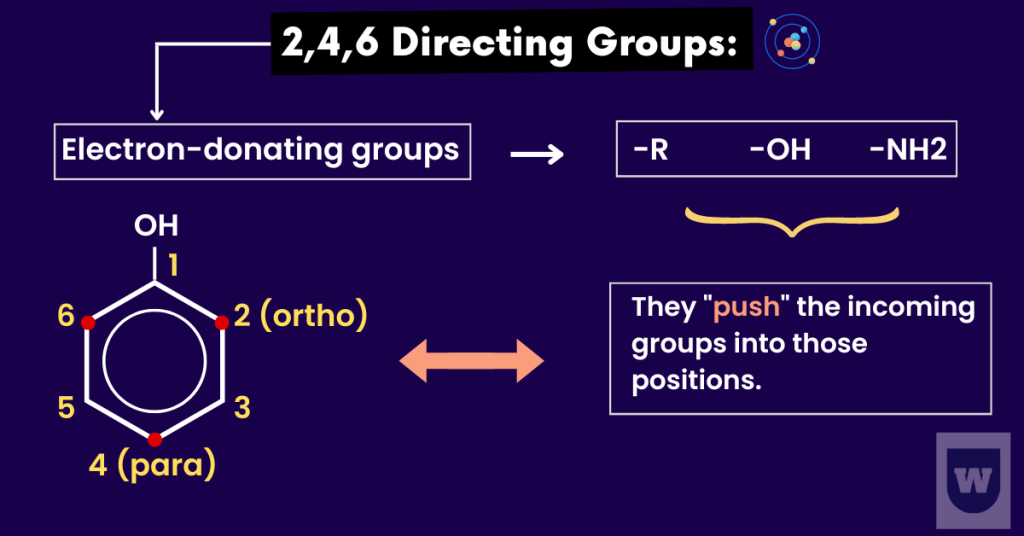
This is because the electron density is greater at these positions. Here’s the summary:
- The electron density is greatest at the 2, 4 and 6 positions.
- So, the substitution takes place at the 2, 4 and 6 positions.
It is also known as the electron-donating group.
3,5 Directing Groups:
On the other hand, some groups (such as CN) direct the incoming groups at position 3 or 5.
They are known as electron-withdrawing groups. The table below shows this:
| Groups that direct the incoming electrophile to the 2- or 4- positions | Groups that direct the incoming electrophile to the 3- position |
|---|---|
| –CH3, –alkyl | –NO2 |
| –NH2, –NHR or –NR2 | -CN |
| –OH or –OR | –CHO, –COR |
| –NHCOR | –NH3 |
| –Cl | –CO2H, –CO2R |
Pretty simple, isn’t it?
Wrapping Up:
So, there you have it.
We discussed hydrocarbons (arenes) in this chapter. To be precise, we looked at the structure of benzene and its reactions.
You should practise plenty of past paper questions to ace this topic. Remember that this topic is extensive. Therefore, do practise constantly and you will improve.
Thank you for reading and staying with me till the end. Stay tuned for more.
Further reading:
