If you want to know about ionization energy in periodic table, you will love this easy-to-understand resource.
In fact, this is the exact resource that I used to learn this topic.
But first, let’s start with a simple introduction.
Ionization energy is the energy that an isolated (gaseous) atom needs to release an electron, resulting in a cation. In simple words, it is the measure of difficulty in removing an electron.
Now, let me introduce some other concepts to you.
Ionization Energy in Periodic Table:
Successive ionization energies:
I have a question for you. What is meant by the first ionisation energy?
The first ionization energy of an element is the energy required to remove the outermost electron from an atom in a gaseous state to form a unipositive (+1) gaseous ion.
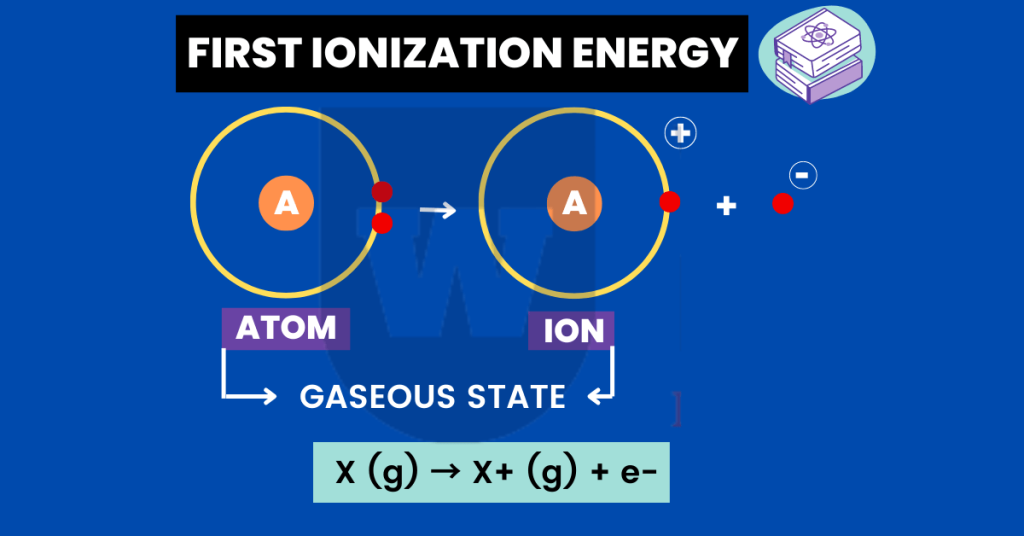
If this sounds confusing to you, do not worry. Let me make this simple for you.
Take a look at the equation below:
Ca (g) → Ca+ (g) + e-
Now, let’s study the above equation step-by-step.
- We have a calcium ion (Ca+). This means that a calcium atom has lost one electron.
In other words, this calcium ion will have to gain one electron to become calcium.
- Note that calcium and its ion are both in a gaseous state.
The energy required to remove this electron from calcium (element) is 590 kJ mol-1. This is what we call the 1st ionization energy of an element.
Pretty simple, isn’t it?
2nd and 3rd ionisation energy:
Let’s continue talking about calcium.
If we now take out one more electron from 1 mole of calcium ion (Ca+), we call it the second ionization energy. Here’s the equation:
Ca+ (g) → Ca+2 (g) + e-
The energy required to remove this electron is 1150 kJ mol-1. So this is the second ionization energy of calcium.
Now, if I remove the third electron from one mole of gaseous 2+ ions, I will need energy of 4940 kJ mol-1. So this will be the third ionization energy. The equation is:
Ca+2 (g) → Ca+3 (g) + e-
Note: We can continue to remove all the electrons. This is what we call successive ionization energy.
From the details above, have you noticed that the successive ionization energies are increasing? Here’s why.
- As more electrons are removed, the charge on the ion gets greater.
Due to this, the size gets smaller and the outermost electrons get closer to the nucleus. This results in a stronger attraction between the positively charged protons (in the nucleus) and electrons.
Here’s one more concept that you should know. Take a look at the image below:
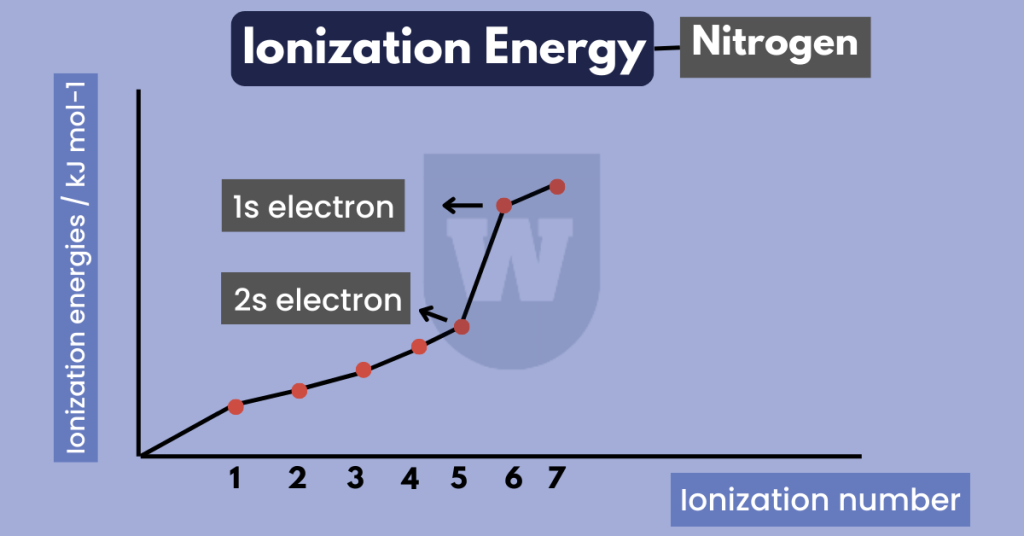
- Notice that there is a big difference between some successive ionisation energies. But why is that so?
For example, there’s a big difference in the 5th and 6th ionisation energy for Nitrogen. This simply shows that the electron is being removed from a principal quantum number closer to the nucleus.
Let me make this simple for you. The electronic configuration of Nitrogen is:
1s22s22p3
The first 5 electrons for Nitrogen are removed from the principal quantum number 2. But the 6th electron is removed from quantum number 1.
Since electrons in quantum number 1 are closer to the nucleus, higher energy is required to overcome the strong attraction between the nucleus and the electrons.
Summary: Ionization energy is the measure of difficulty in removing an electron. You can also say that it is the energy that an isolated (gaseous) atom needs to release an electron, resulting in a cation.
Now you might be wondering, what are the factors that affect ionisation energy?
Factors affecting ionisation energy:
The three main factors that affect I.E are:
- Distance of outer electrons from the nucleus.
- Nuclear charge.
- Shielding effect of inner electrons.
Let me explain all these to you in detail.
Distance of outer electrons from the nucleus:
In simple words, we are going to talk about the size of an atom. Note that size refers to the atomic radii of the atom.
Here’s a question for you.
What happens to the force of attraction between the positive and negative charges as the distance between the increases? It decreases.
So here’s the simple takeaway.
As the size of an atom increases, the outermost electrons get away from the nucleus. So the force of attraction between the positively charged nucleus and the negatively charged electrons decreases.
On the other hand, the valence (outermost) electrons of a smaller atom are closer to the nucleus. Therefore, they experience a greater force of attraction.
Further reading:
3 Types of Intermolecular Forces in Hydrogen Fluoride (HF) | Best Guide
How to find molar mass (with periodic table)? The Ultimate Guide
Covalent bonding in water (H2O) | Must Read
Now, let’s see how nuclear charge affects I.E.
Nuclear charge:
As the number of protons (atomic number) increases, the positive nuclear charge increases.
You should simply take the positive nuclear charge as the force of attraction between the nucleus and the electrons.
So more energy is needed to overcome these attractive forces and remove an electron.
Pretty simple, isn’t it? Now, let’s talk about the shielding effect.
Electron shielding:
This is a fun concept. Let me explain.
This topic simply shows us how the inner electrons “protect” the outermost electrons from the nuclear attraction. Here’s how.
The electrons between the nucleus and the valence (outermost) electrons shield the outermost electrons from the nucleus. This is because all the electrons are negatively charged and they repel each other.
So the greater the shielding, the lower the attractive forces between the nucleus and the outer electrons. So this is how electron shielding decreases the I.E.
In short, here’s a quick summary.
- The further the valence electrons are from the nucleus, the lower the ionisation energy.
- As the proton number increases, ionisation energy also increases (generally).
- As the shielding increases, it is easier to remove the outer electrons
Patterns in ionisation energies in the periodic table:
Before moving on to this topic, here’s a quick recap.
Periods are the horizontal rows (left to right), while groups are the vertical columns (top to bottom). Now, let’s talk about the trends of ionisation energies across a period.
As we move across the period in the periodic table, the ionisation energies (generally) increase. To better understand this topic, take a look at the image below:
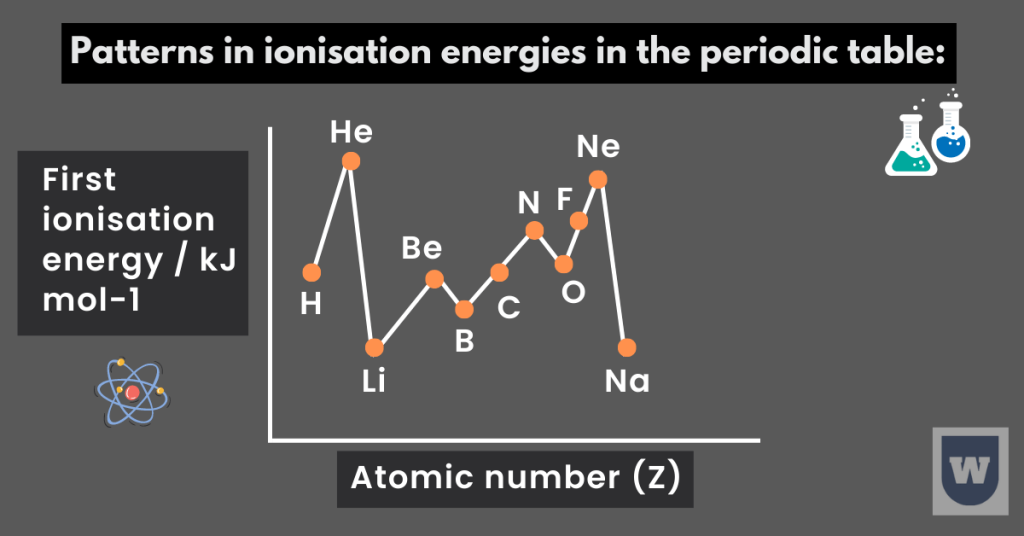
- Notice how the I.E energy increases across period 1 (hydrogen to helium) and period 2 (lithium to neon).
But why is this so?
This is because as we move across the period, the nuclear charge increases. But the shielding by the inner shells remains constant.
Now here’s one more thing to consider.
- Note that there is a rapid decrease in the first I.E between the last element of one period and the first element of the next period. Can you guess why is this so?
Let’s take helium and lithium to better understand this topic.
Helium has two electrons (both in the first quantum shell). On the other hand, lithium has three electrons. You should know that this third electron goes to the next quantum shell (further away from the nucleus).
In short, this happens because:
- The shielding by inner shells increases.
- The distance between the nucleus and the outermost electrons increases.
Now, let’s take a look at some anomalies (deviation from standard).
- The first I.E of boron (atomic number 5) is less than that of beryllium (atomic number 4). Can you guess why is this so?
Let me explain.
The electronic configuration of beryllium is:
1s2 2s2
The electronic configuration of boron is:
1s2 2s2 2p1
So here’s the interesting thing: The fifth electron of boron is in the 2p subshell.
Since the 2p subshell is further away from the nucleus than the 2s subshell, there is less attraction between the nucleus and the fifth electron in boron.
Pretty simple, isn’t it?
- In the same way, the first I.E of oxygen is less than that of nitrogen (refer to the image above).
Here’s the electronic configuration of oxygen and nitrogen:
1s2 2s2 2px2 2py1 2pz1 (oxygen)
1s2 2s2 2px1 2py1 2pz1 (nitrogen)
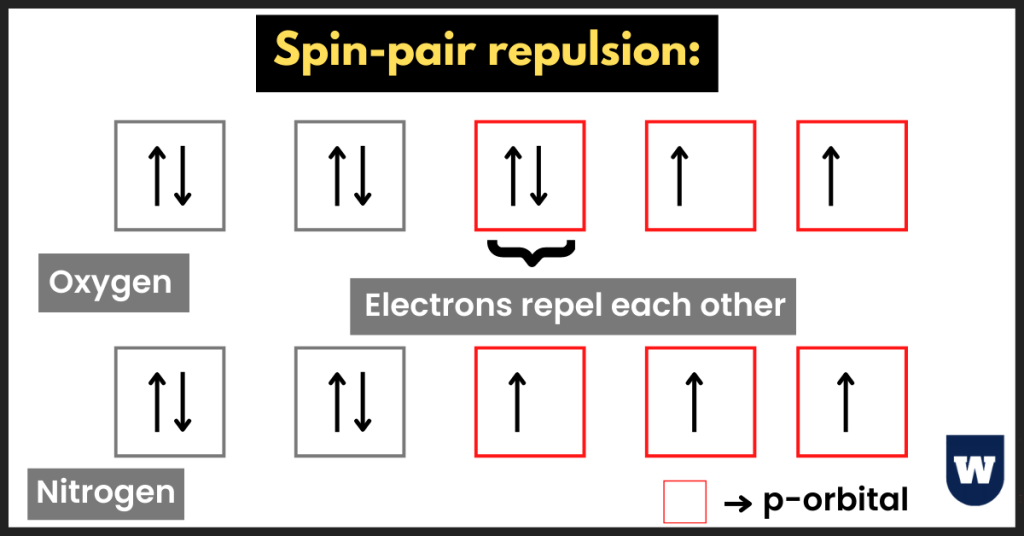
Notice that nitrogen has half-filled orbitals. In oxygen, the presence of the fourth electron disturbs the half-filled orbitals. This makes oxygen “unstable”. So less energy is required to remove this electron.
In other words, the atomic structure for oxygen helps us to understand how the paired electrons in the 2px subshell repel each other (spin-pair repulsion).
Patterns down a group:
As we move across the period, the I.E increases. But what happens when we move down the group?
Let me help you guess this.
Take a look at the first ionization energises down Group 1:
Li: 519 kJ mol-1
Na: 494 kJ mol-1
K: 418 kJ mol-1
Rb: 403 kJ mol-1
From the above information, we can easily guess that ionisation energy (I.E) decreases down the group. Can you tell me why is this so? The reason is simple.
As we move down the group, the size of the atom increases. In other words, we move to a higher principal quantum level down the group.
This is the reason why it gets easier to remove the valence electron as we move down the group.
To summarise, the first I.E decreases down the group as the shielding by inner shells increases. Plus, the distance between the outer electrons and nucleus also increases.
This outweighs the increased nuclear charge, causing I.E to decrease.
Simple, isn’t it?
Wrapping up:
With this, our topic about ionization energy in the periodic table has come to an end.
If you want to improve your grip on this concept, I recommend you practise some questions. And do let me know whether you were able to solve the question above.
Just to recap, I.E increases across the period (generally) and decreases down the group. Thank you for reading and staying with me till the end.
Stay tuned for more.
