If you want to cover complete A Level (AS) inorganic chemistry, you will love this fantastic resource I am about to share with you.
In fact, this exact resource helped me ace inorganic chemistry in my exam.
But before talking about this topic in detail, you should know that there are 4 sub-topics:
- Chemical periodicity
- Group 2
- Group 17
- Nitrogen and sulfur
Overview: Inorganic chemistry is the study of the properties and behaviour of inorganic compounds. To cover this topic, we will be looking at period 3 (periodicity), group 2 metals, group 17 (halogens) and nitrogen and sulfur.
Now let’s talk about each of them in detail.
Inorganic Chemistry
Chemical periodicity (period 3):
As you already know, a period is a row (horizontal) of chemical elements. Plus, all the elements in a period have the same number of electron shells.
Pretty simple?
Now here’s the interesting part.
Period 3 of the periodic table consists of the following elements:
- Sodium (Na), Magnesium (Mg) and Aluminium (Al) → Metals
- Silicon (Si) → Macromolecule
- Phosphorus (P), Sulfur (S), Chlorine (Cl) and Argon (Ar) → (Non-metals)
The first thing you should know is the trend in the melting points of these elements.
Before discussing this topic, I want you to look at the image below:
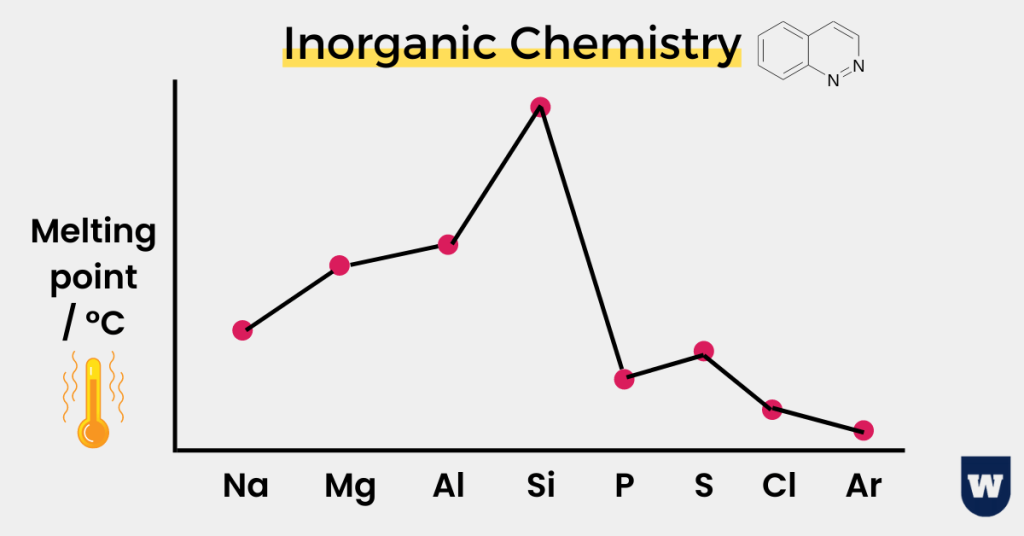
So here’s the takeaway.
Concept 1:
The melting point increases from Na to Al.
This is because the metal lattice structure gets bigger due to the increasing proton number. In simple words, the attraction between the positive ions and delocalised electrons in Al is more than Na.
Therefore, the melting point of aluminium is higher than that of Na.
Concept 2:
Now retake a look at the graph. What else do you notice?
There is a big jump in the melting point from aluminium to silicon. But why is that so?
Silicon is a macromolecule due to the linking of atoms in a tetrahedral structure. Therefore, a lot of energy is required to overcome the strong covalent bonds.
And the mutual sharing of electrons (covalent bonding) also makes these bonds stronger.
Note: You can be asked to draw a diagram for the period 3 elements melting points as well!
Concept 3:
The melting point decreases from Phosphorus to Argon. But here’s one concept that you should know.
The melting point of sulfur is higher than that of phosphorus. Can you guess why is this so?
This is because phosphorus exists as P4 molecules while sulfur consists of S8 rings of atoms. As a result, the greater number of electrons in sulfur leads to strong Van der Waals forces.
This explains why the melting point of phosphorus is lower than that of sulfur.
Pretty simple, isn’t it?
Now, let’s take a look at some reactions.
Reactions of period 3 elements with Oxygen:
In general, you should know that when an element will react with oxygen, an oxide will be formed.
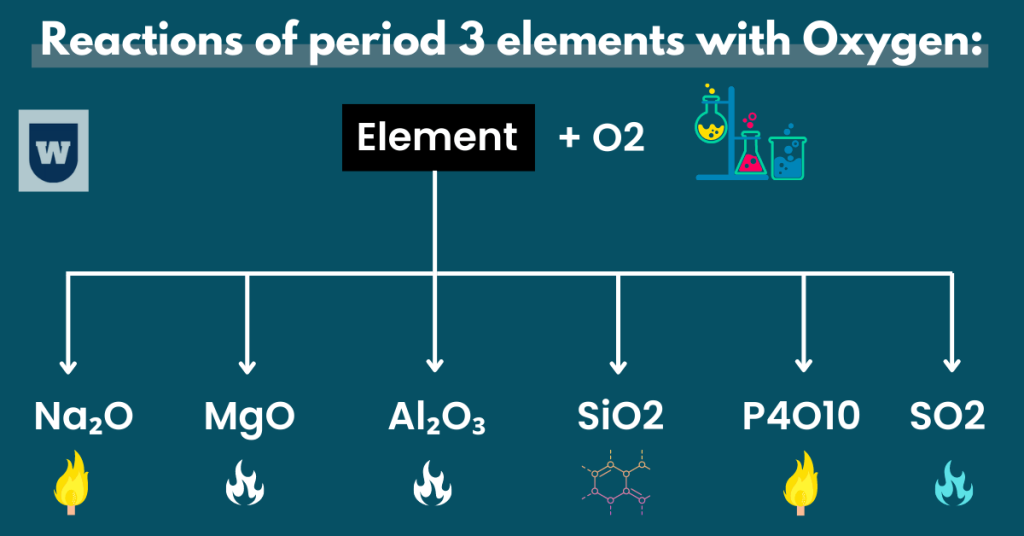
So here are all the reactions you should learn:
2 Na + 1/2 O2 → Na₂O
In this reaction, a bright yellow flame can be observed. Apart, sodium is a very reactive metal. So it reacts with oxygen to form sodium oxide which is an unstable compound.
Mg + 1/2 O2 → MgO
In this reaction, a bright white flame can be observed (as much heat is released). Note that powdery magnesium oxide (MgO) is formed during this reaction.
2 Al + 3/2 O2 → Al₂O₃
When aluminium (a grey metal) reacts with oxygen, aluminium oxide (white solid) is formed. Note that a bright white flame can be observed during this reaction.
Si + O2 → SiO2
When silicon reacts with oxygen, silicon dioxide (a macromolecule) is formed. You should know that this reaction is slow as the thin layer of silicon dioxide “protects” silicon.
P4 + 5 O2 → P4O10
Diphosphorus pentaoxide is formed when solid phosphorus reacts with oxygen (gaseous). Now here’s something that you should know.
P2O5 and P4O10 are the same compounds but the different forms. In simple words, P2O5 is the empirical formula while P4O10 is the molecular formula.
Note: We use P4O10 as it has a higher oxidation state. Apart, a white or yellow flame can be observed during this reaction.
1/8 S8 + O2 → SO2
When sulfur (a pale yellow solid) reacts with oxygen, sulfur dioxide is formed. You should note that a blue flame colour is observed in this reaction.
Pretty simple?
This takes us straight to our next topic.
Reactions of period 3 elements with chlorine:
Here are some reactions of period 3 elements with chlorine that you should know.
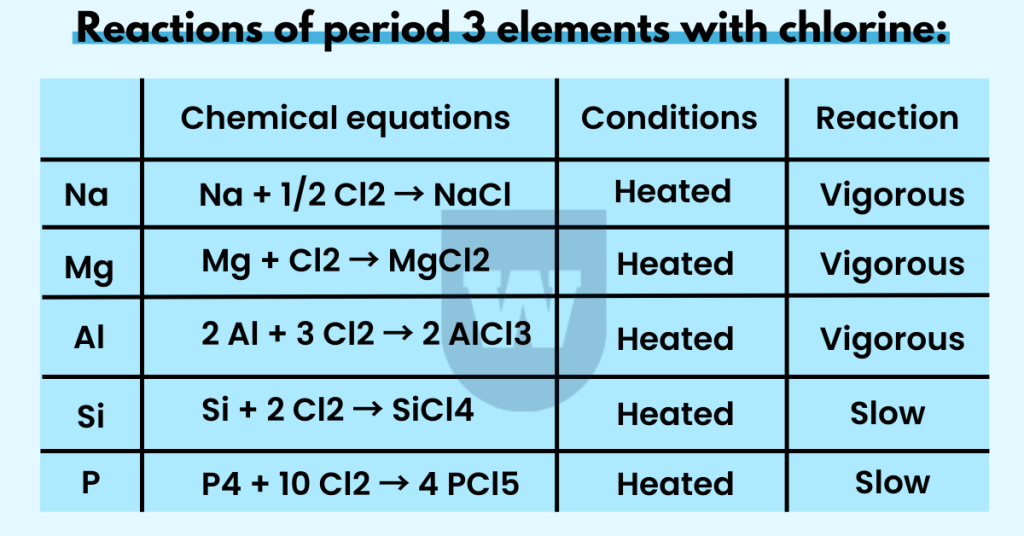
Na + 1/2 Cl2 → NaCl
You should know that when sodium (grey metal) reacts with chlorine (green gas), sodium chloride (white solid) is formed. Plus, this is a vigorous reaction and NaCl is an ionic compound.
Mg + Cl2 → MgCl2
Magnesium chloride is also an ionic compound, and a vigorous reaction occurs when the elements are heated. You should also know that magnesium chloride dissolves in water. This gives a faintly acidic solution as the pH is around 6).
Now let’s talk about aluminium.
2 Al + 3 Cl2 → 2 AlCl3
At room temperature, aluminium and chlorine do not react rapidly. However, if aluminium is heated under anhydrous conditions, the reaction is vigorous.
As a result of this reaction, aluminium chloride is formed. Note that it is a white powder. Now here’s something interesting for you.
AlCl3 is a covalent compound that turns into a dimer when heated (Al2Cl6). Therefore, it acts as a simple molecular chloride.
Si + 2 Cl2 → SiCl4
If we pass chlorine gas over silicon powder (heated), silicon tetrachloride is formed. This is a colourless liquid and the reaction is slow.
P4 + 10 Cl2 → 4 PCl5
For this reaction, you should know that PCl3 (phosphorus trichloride) is formed in a limited halogen. But in excess halogen, PCl5 (phosphorus pentachloride) is formed.
Reaction with water and Acid-Base behaviour:
Now here are some other important reactions that you should know about:
(Reactions of metals and their oxides with water).
Sodium (Na):
2 Na + 2 H2O → 2 NaOH + H2
Na2O + H2O → 2 NaOH
Here are some concepts you should know:
Metal oxides: Basic in nature
Non-metal oxides: Acidic in nature
Note that sodium hydroxide (NaOH) is very alkaline as its pH is around 13. Plus, when metals react with water, we get a hydroxide and hydrogen gas.
But when metal oxides react with water, we get hydroxides only. Now let’s talk about magnesium.
Magnesium (Mg):
Mg(s) + 2 H2O(l) → Mg(OH)2 (aq) + H2(g)
MgO + H2O → Mg(OH)2
Here’s something interesting that you should know.
Water has NO effect on magnesium metals at room temperature as magnesium is a slow reacting element. But, magnesium forms magnesium hydroxide and hydrogen gas when it reacts with water vapour.
Now I have a question for you. Why magnesium does not react with cold water?
This is because it is coated with insoluble magnesium hydroxide. So the water does not come in contact with it. Pretty simple, isn’t it?
Aluminium (Al):
Al2O3 + 6 HCl → 2 AlCl3 + 3 H2O
Al2O3 + 2 NaOH + 3 H2O → 2 NaAl(OH)4
Aluminium oxide is amphoteric. Now, what does this mean?
This means that aluminium oxide is capable of reacting either as an acid or as a base. In short, when aluminium oxide reacts with HCl (acid), it acts as a base.
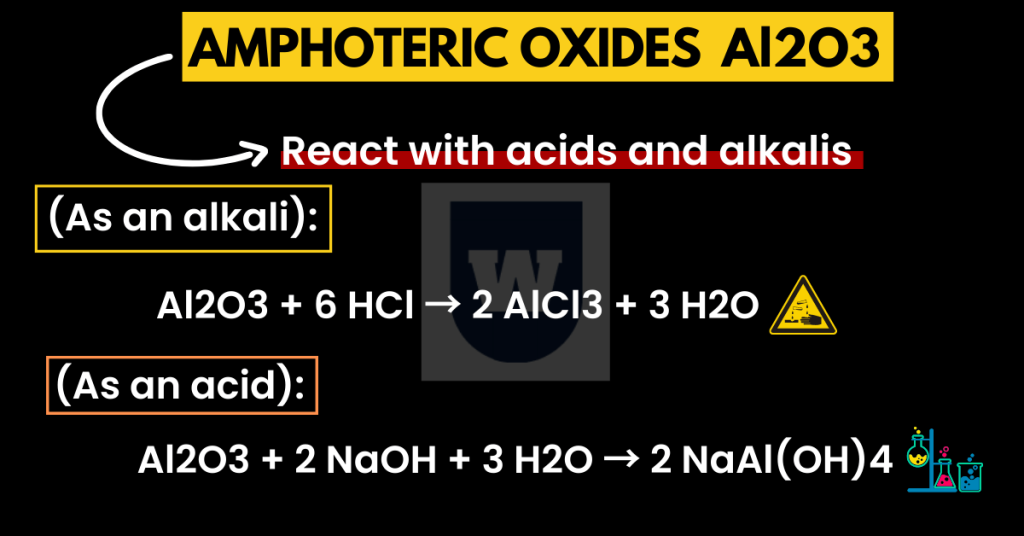
But when aluminium oxide reacts with sodium hydroxide (base), it acts as an acid. This is what we call the ‘acid-base behaviour‘.
Silicon (Si):
Do you know that silicon dioxide does NOT react with water as it is difficult to break the giant covalent structure?
Therefore, we talk about the acid-base behaviour of silicon dioxide. Recall that non-metals are acidic in nature.
SiO2 + 2 NaOH → Na2SiO3 + H2O
In this reaction, silicon dioxide reacts with hot and concentrated sodium hydroxide to form colourless sodium silicate.
Further reading:
3 Types of Intermolecular Forces in HF (Hydrogen Fluoride)
Atomic structure for Oxygen explained | With diagrams
Now, let’s talk about phosphorous.
Phosphorus:
P4O10 + 6 H2O → 4 H3PO4
P4O10 + 12 NaOH → 4 Na3PO4 + 6 H2O
Note that when phosphorus (V) oxide reacts violently with water. As a result, we get a solution containing a mixture of acids. But, you only need to consider one of these – Phosphoric (V) acid (H3PO4).
You should also know that H3PO4 is a weak acid.
When phosphorus (V) oxide reacts with sodium hydroxide, it gives Trisodium phosphate (Na3PO4). As a result of this reaction, we can also get some other salts. But you can only know the one I stated above.
Sulfur:
For this part, we will be looking at sulfur dioxide (SO2) and sulfur trioxide (SO3).
SO2 + H2O → H2SO3
SO3 + H2O → H2SO4
SO2 + 2 NaOH → Na2SO3 + H2O
If you look at the third reaction, sulfur dioxide acts as a base. As a result of this reaction, sodium sulfite solution is formed.
Pretty simple, isn’t it?
The reaction of period 3 chlorides with water:
Here are some important reactions that you should know about.
| Sodium chloride | NaCl (s) → Na+ (aq) + Cl- (aq) (dissolves) |
| Magnesium chloride | MgCl2 (s) → Mg2+ (aq) + 2Cl- (aq) (dissolves) |
| Aluminium chloride | AlCl3 + 6 H2O → [Al(H2O)6]^3+ + 3Cl- |
| Silicon tetrachloride | SiCl4 (l) + 2 H2O (l) → SiO2 (s) + 4 HCl (g) |
| Phosphorus pentachloride | PCl5 (l) + 4 H2O (l) → H3PO4 (aq) + 5HCl (g) |
(Some other period 3 important concepts):
- The atomic radius decreases across the period. Here’s why.
The shielding remains (almost) constant but the nuclear charge increases. This is because the electrons and protons increase.
So due to a greater attraction between the nucleus and the outermost electrons, the atomic radius decreases.
- The ionic radius decreases across the period.
Here are two concepts that you should consider.
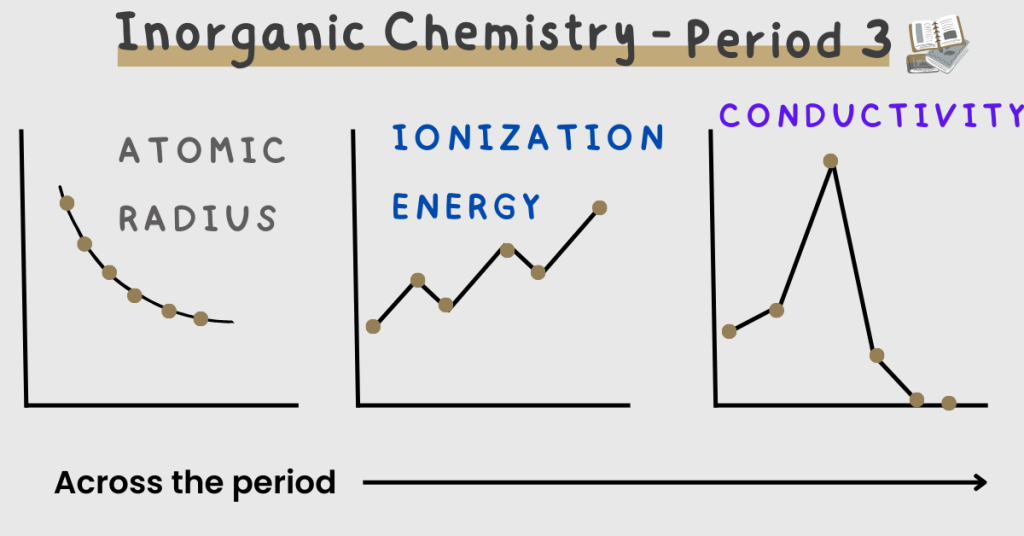
Cations (ionic radius decrease):
Na+ > Mg2+ > Al3+ …
Anions (ionic radius decrease):
P3- > S2- > Cl- …
Group 2:
The first concept that you should know is that ionisation energy decreases down the group.
Can you guess why? Let me explain.
This is because the distance of outer electrons from the nucleus increases. Plus, the increased shielding (the screen effect) reduces the nuclear attraction for electrons.
Before moving on to the reactions, here are two concepts you should know first.
- The melting point decreases down the group. Can you tell me why?
If we move down the group, the atomic size increases. Moreover, the electron shells increase down the group but the number of delocalised electrons per atom remains the same.
So the attraction between the nucleus and the outermost electrons decreases. As a result, the melting point decreases down the group. Simple?
- The reactivity increases down the group. Here’s why.
As we move down the group, the atomic radius becomes larger due to the increased number of shells. Due to this, electrons are less tightly held by the nucleus.
But why is this important?
Look, this is the reason why electrons are easily lost down the group (from Mg to Ba) to form cations. So the reactivity increases. Now let’s move on to the reactions.
Reactions of Group 2 metals with oxygen:
Mg + 1/2 O2 → MgO
When solid magnesium (silver-grey element) reacts with oxygen, magnesium oxide (white oxide) is formed. The flame colour for this reaction is white.
Ca + 1/2 O2 → CaO
As you can see from the above equation, calcium oxide (quicklime) is formed which is an alkaline substance. Plus, a red flame is observed during this reaction.
Sr + 1/2 O2 → SrO
A crimson (red) flame is observed during this reaction.
Ba + 1/2 O2 → BaO
A pale green flame is observed during this reaction. Now, let’s talk about the reactions of Group 2 elements and their oxides with water.
Reaction with water:
Note that group 2 metals react with water to form hydroxides and hydrogen gas. The reactions are summarised below:
Mg + 2 H2O → Mg(OH)2 + H2
For the above reaction, note that the reaction soon stops. This is because magnesium hydroxide is almost insoluble in water. Therefore, it acts as a barrier to magnesium.
(For calcium, strontium and barium):
X + 2 H2O→ X(OH)2 + H2
Here, X is a group 2 element. For this part, you just need to know that these metals react with cold water vigorously.
For example, when calcium reacts with water, bubbles of hydrogen gas are given off. Moreover, a white precipitate of calcium hydroxide forms.
With this, it is time to talk about the sulfates and hydroxides of group 2 elements.
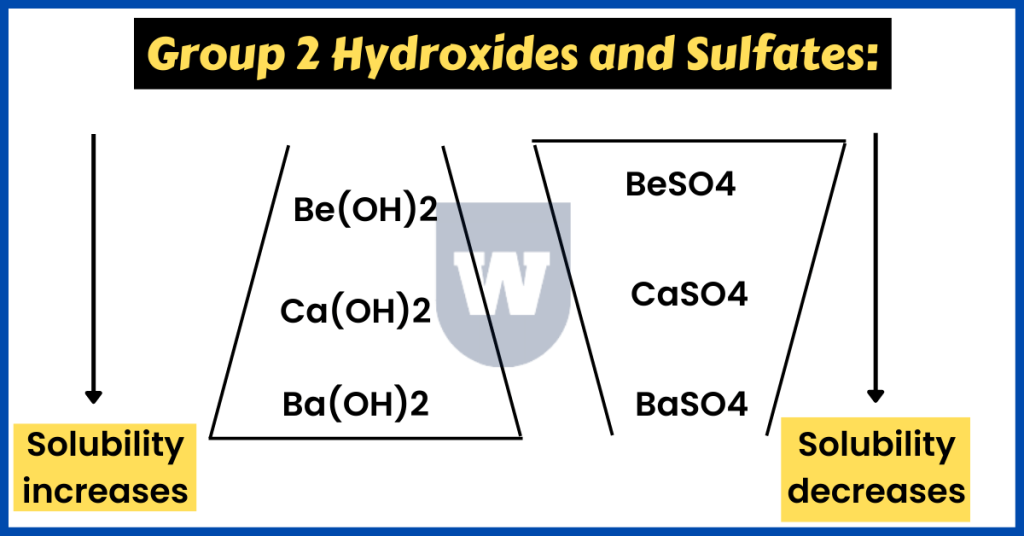
- The solubility of hydroxides increases down the group.
You do not need to know exactly why this happens. But this is due to the decreased lattice energy down the group. So here’s a quick summary of this.
Ba(OH)2 > Sr(OH)2 > Ca(OH)2 > Mg(OH)2
- The solubility of sulfates decreases down the group.
Let me explain this with the help of an example. What would you observe if you mixed radium hydroxide solution with dilute sulfuric acid?
In this reaction, radium sulfate will be formed. Since the solubility of sulfates decreases down group 2, we can expect a white precipitate of radium sulfate to be formed.
The trend in thermal stability of carbonates and nitrates:
This concept is simple.
The carbonates and nitrates of Group 2 elements become MORE thermally stable down the group. Note that being thermally stable means that more temperature (heat) is required to break the bond.
But what happens when carbonates and nitrates decompose?
- Carbonate → Oxide + Carbon dioxide
Here is an example.
MgCO3 → MgO + CO2
- Nitrate → Oxide + Nitrogen dioxide + Oxygen
Here is an example:
2 Ca(NO3)2 → 2 CaO + 4 NO2 + O2
Reactions of Group 2 carbonates with water and dilute acids:
For this topic, the first thing that you should know is that the carbonates of magnesium, calcium, strontium and barium are insoluble in water.
But, they react with dilute acid to produce salt, water and carbon dioxide gas. For example,
MgCO3 + H2SO4 → MgSO4 + H2O + CO2
Note that the magnesium sulfate formed in this reaction is soluble in water. So it remains in an aqueous solution. At the same time, recall that the solubility of group 2 sulfates decreases down the group.
Can you guess the products if the above reaction was carried with dilute nitric acid?
With dilute nitric acid (HNO3), the nitrate salts formed are soluble in water. For example:
CaCO3 + 2 HNO3 → Ca(NO3)2 + H2O + CO2
Group 17:
In inorganic chemistry, we have to study group 17 elements known as “halogens”.
Before moving on to their reactions, let’s talk about their physical properties.
(Physical properties):
The first thing you need to know is that halogens are non-metals. Plus, they exist as diatomic molecules at room temperature. Now, can you guess the colours of some halogens?
Here is what you should know:
Fluorine (F2): Pale yellow
Chlorine (Cl2): Greenish yellow
Bromine (Br2): Red-brown
Iodine: Black (solid) or purple (vapours)
With this, it is time to talk about the melting and boiling points of halogens. You should know that the melting and boiling point increases down the group.
This is because as you move down the group, the number of electrons increases. Due to this, there is a greater chance for instantaneous dipoles to form within the molecules.
Moreover, the colours of halogens get darker as you move down the group. Now, let’s talk about their reactions.
Halogens as oxidising agents:
Do you know that halogens behave as strong oxidants (oxidising agents)? Can you guess why?
Recall that an oxidising agent is reduced itself. Since halogens gain electrons to become stable, they are oxidising agents. But, what is the trend?
As you move down the group, the oxidising strength of halogens decreases. This means that chlorine is a stronger oxidising agent than iodine.
But what about halides (halogens with a negative charge)?
For halides, the reverse is true. Halides are reducing agents and as you move down the group, the reducing strength of halides increases.
This means that iodide (I-) is a stronger reducing agent than chloride (Cl-). Now, let’s talk about some reactions.
Displacement reactions:
You should always remember that a more reactive halogen can replace a less reactive halogen. Plus, the reactivity decreases as you move down the group (17).
Here is an example:
Cl2 + 2 NaBr → 2 NaCl + Br2
In the reaction above, chlorine displaces bromine from its salt. The solution changes to a yellowish-brown colour due to dissolved bromine molecules.
You should know that the reaction takes place as chlorine atoms are more electronegative than bromine atoms. In the same way, bromine can displace iodine from an iodide solution.
Testing for halide ions:
This is an extremely important topic.
We can detect halide ions using silver nitrate solutions. We then add a few drops of dilute nitric acid to acidify the test solution. The table below summarises this:
| Halide ion | Colour of silver halide precipitate on addition of silver nitrate solution | Effect on the precipitate of adding dilute ammonia solution | Effect on the precipitate of adding concentrated ammonia solution |
| Chloride (Cl-) | White | Dissolves | Dissolves |
| Bromide (Br-) | Cream | Insoluble (Partially soluble) | Dissolves |
| Iodide (I-) | Pale yellow | Insoluble | Insoluble |
Note that you can be asked to draw the above table.
Reactions of halide ions with concentrated sulfuric acid:
Do you know that compounds containing Cl-, Br- and I- ions will react with concentrated sulfuric acid? Here is what you need to know.
- NaCl + H2SO4 → NaHSO4 + HCl
In the above reaction, HCl produced is visible as white fumes. Note that concentrated sulfuric acid is a strong oxidising agent. But it is NOT strong enough to oxidise HCl.
Therefore, no further reaction takes place.
- NaBr + H2SO4 → NaHSO4 + HBr
(followed by oxidation of HBr:)
2 HBr + H2SO4 → Br2 + SO2 + 2 H2O
Note that a reddish-brown gas (Br2) is observed during this reaction.
- NaI + H2SO4 → NaHSO4 + HI
(followed by oxidation of HI:)
2 HI + H2SO4 → I2 + SO2 + 2 H2O (violet vapours of I2 are observed)
And:
6 HI + H2SO4 → 3 I2 + S + 4 H2O (Yellow solid of S is observed)
And:
8 HI + H2SO4 → 4 I2 + H2S + 4 H2O (strong, bad smell of H2S)
Disproportionation:
Let me make this simple for you. The element chlorine has an oxidation number of 0. But it undergoes a type of redox reaction (disproportionate) where it is oxidised as well as reduced.
The two equations below will help you understand this.
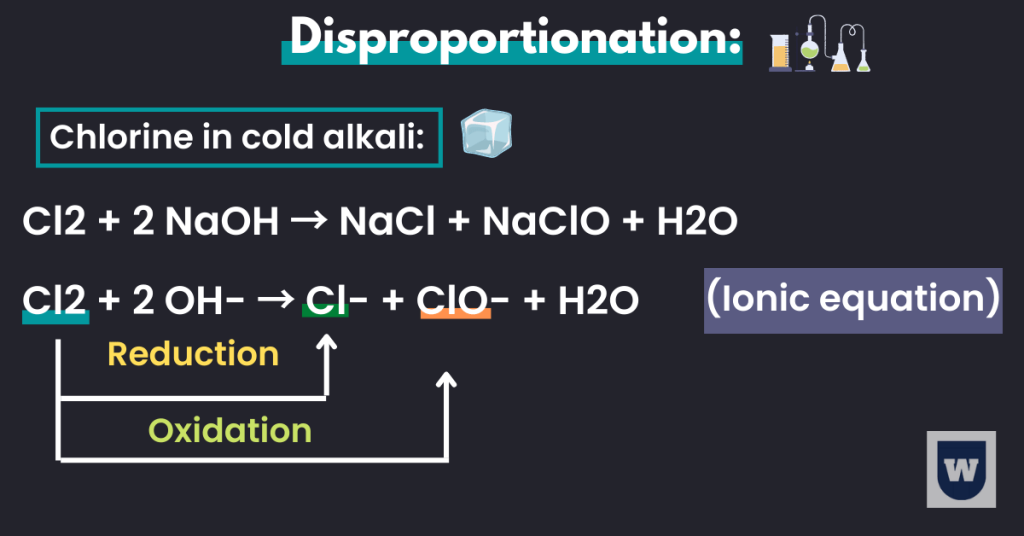
Chlorine with hot alkali:
3 Cl2 + 6 NaOH → 5 NaCl + NaClO3 + 3 H2O
The ionic equation for the above reaction is:
Cl2 + 6 OH- → Cl- + ClO3- + 3 H2O
When chlorine reacts with hot alkali, the oxidation number changes from 0 to -1 (reduction), and from 0 to +5 (oxidation). This is what we call disproportionation.
Pretty simple, isn’t it?
Nitrogen and sulfur:
I have a question for you? Why is nitrogen unreactive?
Since nitrogen needs 3 electrons to complete its outermost shell, it forms a strong triple covalent bond. It is difficult to break this bond. So nitrogen only reacts under extreme conditions.
Plus, nitrogen is non-polar.
Now let’s talk about ammonia.
(Ammonia):
Ammonia (NH3) is an alkaline gas and is formed during the Haber process. The reaction is:
N2 + 3 H2 ⇌ 2 NH3
Ammonium compounds are important fertilizers and they replace nitrogen in the soil. Some ammonium salts used in fertilizers are:
- NH4Cl
- NH4NO3
- (NH4)2SO4
But why am I telling all this to you? Here’s the concept that you need to know.
The excess fertilisers can be leached out of soils into rivers and lakes, where it causes eutrophication. This kills aquatic life.
Now let’s move on to some equations.
N2 + O2 → 2 NO
2 NO + O2 → 2 NO2 (Nitrogen (II) oxide is being oxidised)
The nitrogen (IV) oxide formed then dissolves in water droplets to form nitric acid:
2 NO2 + H2O + 1/2 O2 → 2HNO3
Note that nitrogen oxides are pollutants. As you can see from the reactions above, they cause acid rain.
Moreover, photochemical smog is caused when unburnt hydrocarbons react with nitrogen dioxide. This happens due to a pollutant known as Peroxyacetyl nitrate (PAN).
But, do you know that oxides also catalyse the oxidation of sulfur dioxide gas? Here are the equations:
SO2 + NO2 → SO3 + NO
NO + 1/2 O2 → NO2
The above equations show that nitrogen dioxide (NO2) acts as a homogenous catalyst.
Here’s how oxides of sulfur contribute to acid rain:
SO2 + H2O → H2SO3
SO3 + H2O → H2SO4
We know that acid rain damages plants, rivers, lakes, streams and buildings. Moreover, it leaches nutrients from the soil.
But, do you know that car exhaust systems use catalytic converters to reduce pollutants. The equation below shows how it’s done.
2 CO + 2 NO → 2 CO2 + N2
Wrapping up:
With this, our topic about inorganic chemistry for A levels has come to an end. Now I turn it over to you.
Which concept of inorganic chemistry do you find challenging? Or there’s a one you find interesting? Either way, do let me know.
You should practise plenty of past paper questions to ace this topic. Remember that this topic is extensive. Therefore, do practise constantly and you will become comfortable with it.
Thank you for reading and staying with me till the end.
