If you want complete alternative to practical (ATP) notes for GCE O Level Chemistry (5070), you will love this comprehensive resource I am about to share with you.
In fact, this is the resource I used to ace my ATP exam.
But before moving ahead, note that this written paper consists of multiple (compulsory) short answer questions (along with structured questions). This paper tests your knowledge about laboratory practical procedures.
Now let’s start without further introductions.
Alternative to Practical (ATP) Notes:
In this exam, you will have questions such as:
- Recording readings of apparatus (from diagrams)
- Suggest, describe and explain “experimental arrangements“, procedures and techniques
- Plot graphs and complete tables
- Using graphs to draw conclusions
- Describing tests for ions, gases and oxidising and reducing agents
I will show you (with examples) how to solve these questions. But before that, here is some basic knowledge about laboratory apparatus you should know.
Laboratory Apparatus:
Below are some details (and uses) of some common laboratory apparatus:
- Measuring cylinder: It is also known as a graduated cylinder and is used to measure the volume of a liquid (not accurate).
- Beaker: A container (cylindrical in shape) for holding liquid and solid samples for experiments.
- Conical flask: It is also known as a titration flask and is used to make solutions (for reactions) or collecting samples.
- Test tube: They are used for reactions and handling chemicals. They can be also used for heating solids and liquids for a reaction (but in a small amount).
- Gas syringe: This apparatus is used to add or remove gas from a (closed) system. Moreover, it is also used during experiments to find out the volume of gas released (which allows to find out the rate of reaction).
- Burette: It is used to measure the accurate volume of liquids (up to 50 cm3). It is used during experiments to get precise readings (but is slightly less accurate than a pipette).
Some other apparatus you should know about are:
- Evaporating dish: This wide and shallow apparatus allows liquids to evaporate. In simple words, it is used for the evaporation of solutions.
- Bunsen burner: This gas burner provides heat during an experiment to substances (also used for combustion).
- Volumetric flask: It is used to prepare precise solutions very accurately (of known concentration).
These were some basic uses of the most commonly used apparatus in the laboratory. Now, let’s move on to the topics which appear commonly in CIE ATP exams.
General rules of solubility (for common salts):
Below is a table that summarises the solubilities of some salts in water.
Note: It is important to know the solubilities of some salts because their preparation is a part of your syllabus.
| No. | Anions | Solubility in Water |
|---|---|---|
| 1. | Nitrates (NO3 -) | All are soluble |
| 2. | Halides: Bromide (Br -) Chloride (Cl -) Iodide (I -) | All are soluble except lead (Pb2+) and silver (Ag+) |
| 3. | Carbonates (CO3 2−) | They (all) are insoluble except K+, NH4+ and Na+ |
| 4. | Sulphates (SO42−) | All are soluble except Pb2+, Ba2+, Ag+ and Ca2+ |
| 5. | Oxides & Hydroxides | All are soluble except K+ and Na+ |
| 6. | Cations | All K+, Na+ and NH4+ salts are soluble |
When you know about the solubilities of salts, it will be easier to learn the topic about the preparation of salts (because the method to prepare soluble and insoluble salts is different).
With this, it is time to move on to another very important topic for the ATP paper, which is about the identification of cations and anions.
Identifications of cations:
This is a part of qualitative analysis (salt analysis) and is one of the most important topics for this paper. Therefore, the table below will help you to memorise some important details.
This table is about the effect of aqueous sodium hydroxide and aqueous ammonia on cations. (ppt refers to precipitate in the table).
Some points regarding the identifications of cations (positive ions) are:
- They (cations) can be identified by the (colour) precipitate.
- If we add excess NaOH (sodium hydroxide), then some precipitates may dissolve to form a colourless solution.
- The same is done with aqueous ammonia.
| Cations | Effect of NaOH (aq) | Effect of NH3 (aq) |
|---|---|---|
| Aluminium (Al 3+) | White ppt (soluble in excess) | White ppt (insoluble in excess) |
| Calcium (Ca 2+) | White ppt (insoluble in excess) | No ppt |
| Zinc (Zn 2+) | White ppt (soluble in excess) | White ppt (soluble in excess) |
| Copper (Cu 2+) | Light blue ppt (insoluble in excess) | Light blue ppt (soluble in excess) |
| Iron || (Fe 2+) | Green ppt (insoluble in excess) | Green ppt (insoluble in excess) |
| Iron ||| (Fe 3+) | Reddish-brown ppt (insoluble in excess) | Reddish-brown ppt (insoluble in excess) |
| Ammonium (NH 4+) | Ammonia gas released on heating |
Now, let me share another important topic with you, which is about the identification of anions (negative ions).
Identification of anions:
Some of the anions you should know about are:
- Carbonate ion (CO3-2)
- Sulphate ion (SO4-2)
- Nitrate ion (NO3-)
- Chloride ion (Cl -)
- Iodide ion (I -)
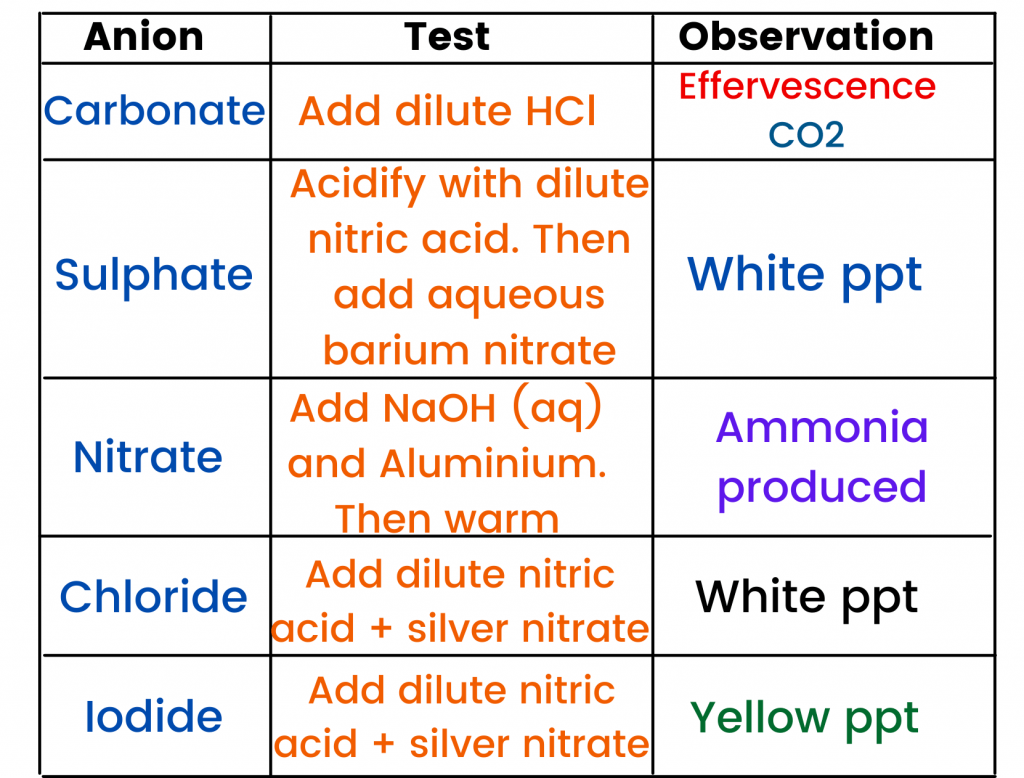
The table above will help you to learn about the tests (and observation) for the anions required by your syllabus.
With this, let’s move on to another very important topic which is about the tests for some gases.
Further Reading:
Chemical Bonding | O Level Questions
Electrolysis explained in plain English | O Level
Organic Chemistry Made Simple | O Level
Test for Gases:
You should know that a gas is often released (given off) during the test of an unknown salt.
Below are some details for you about the tests for some gases. Remember that this topic is important for your Chemistry exam. Therefore, try to learn them.
Carbon Dioxide (CO2) → Bubble it through limewater (it turns milky). A white precipitate is formed.
Hydrogen (H2) → Place a lighted splint. It goes off with a “pop” sound.
Oxygen (O2) → It relights a glowing splint.
Ammonia (NH3) → It turns moist red litmus paper blue.
Chlorine (Cl2) → It turns moist blue litmus paper red (because it is acidic in nature), and then is bleached.
Sulfur Dioxide (SO2) → It turns purple acidified Potassium Manganate (V||) colourless.
How to solve Moles questions in ATP?
In the alternative to practical (ATP) exam, the questions related to moles are “must“. This means that in almost all papers, moles related questions appear.
Note that these questions carry a lot of marks (and you have to practise them to get good grades).
Below I have solved a complete past paper (moles) question (so you know how to solve them). Therefore, try to understand the concept so that you can solve questions like this easily.
Question 2 (May June 2020):
Limewater is a saturated solution of Ca(OH)2 (calcium hydroxide).
The mass of calcium hydroxide in 1.00 dm3 of limewater is found out by a student. The student takes the following steps:
- 25.0 cm3 of limewater is measured (in a flask) using a 100 cm3 measuring cylinder.
- Methyl orange indicator is added to the flask.
- 0.100 mol/dm3 HCl is added to a burette and the initial reading is noted.
- The HCl is added to the flask until the mixture changes colour.
- He then notes down the final reading.
- He repeats the same experiment twice.
(a): The diagram below shows the initial and final titration results (I have completed the below table for you).
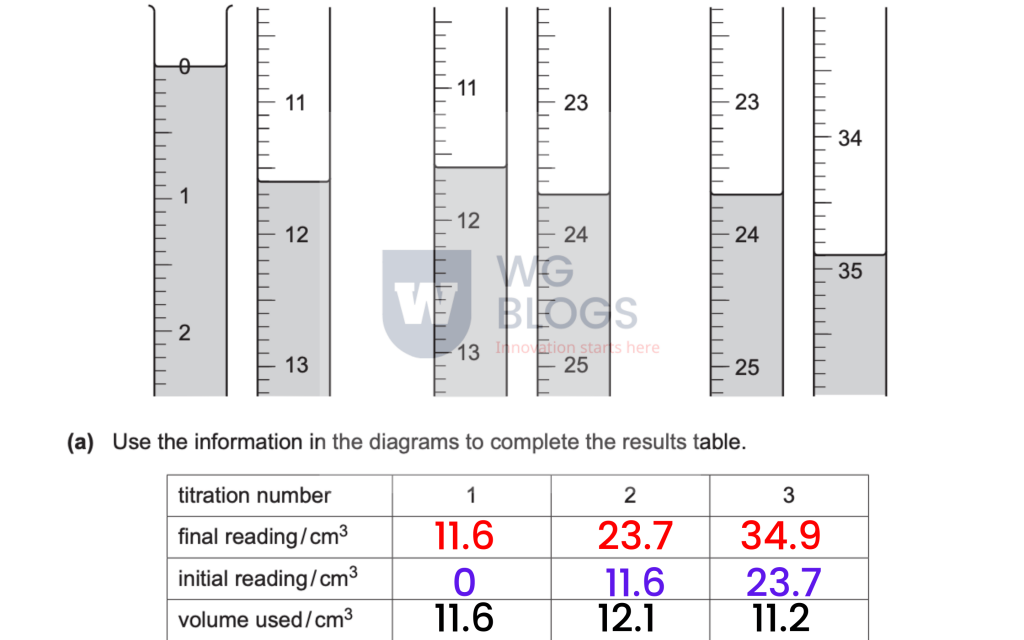
(b) (i): The results were not consistent.
Identify the apparatus that the student may have used (that is not accurate enough) for the titration.
Answer: Measuring cylinder (because it does not give accurate readings).
(b) (ii): Suggest a more accurate piece of apparatus for the experiment (that can be used for the experiment).
Answer: Pipette or Burette because they are more accurate than the measuring cylinder.
(b) (iii): The student adds HCl (hydrochloric acid) drop-by-drop near the endpoint of the titration. Suggest why the student adds HCl drop by drop?
Answer: This ensures that the experiment is more accurate. This is because if the experiment is done in this way, it will prevent going past the endpoint.
Lastly, it will ensure that the correct volume (amount) of neutralisation is measured.
(c): The exact experiment is repeated by the student (three more times). The results are shown in the table below. Tick the best titration result.
Note: The best titration results are those results that are closer to each other (I have completed the table for you).
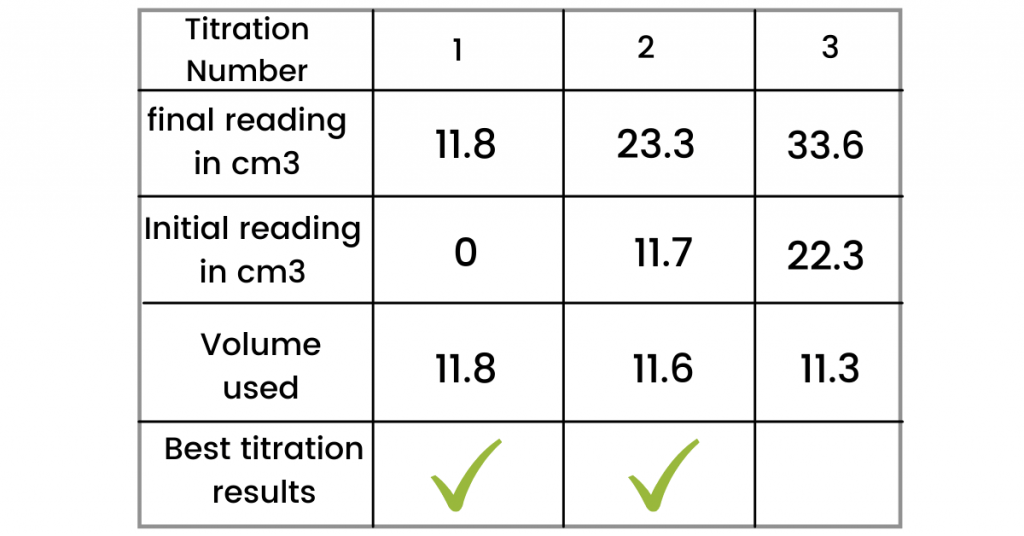
(i): Use the ticked values (from the table above) and calculate the average volume of 0.100 mol/dm3 the hydrochloric acid (HCl) used.
Answer: Average volume = (11.8 + 11.6) / 2 = 11.7 cm3
(ii): Find out the number of moles of the HCl used in the average volume of 0.100 mol/dm3 HCl.
Answer: Number of moles = (Concentration) x (Volume)
So moles = 0.100 x (11.7 / 1000) = 1.17 x 10-3 mol
Note: We divided 11.7 with 1000 to convert cm3 to dm3 (you should look at the units very carefully).
(iii): Calculate the number of moles of Ca(OH)2 (calcium hydroxide) in 25.0 cm3 of limewater by using the equation below:
2 HCl + Ca(OH)2 → CaCl2 + 2 H2O
Answer: Using the equation, we can say that the ratio of the number of moles of HCl and Ca(OH)2 is 2:1.
This means that the number of moles of Calcium hydroxide will be half the number of moles of hydrochloric acid (HCl).
Therefore, the number of moles will be equal to:
(1.17 x 10-3) / 2 = 5.85 x 10-4 mol
(iv): Calculate the number of moles of calcium hydroxide in 1.00 dm3 of limewater.
Answer: Number of moles of Ca(OH)2 in 25.0 cm3 of limewater = 5.85 x 10-4
So the number of moles in 1.00 dm3 (or 1000 cm3) of limewater will be equal to:
= (5.85 x 10-4 x 1000) / 25 = 0.0234 moles
(v): Calculate the Mr (relative molecular mass) of Ca(OH)2.
Answer: To find the Mr, we are going to add relative atomic mass of all the atoms.
Mr = 40 + (2×16) + (2×1) = 74
(vi): Calculate the mass of Ca(OH)2 in 1.00 dm3 of limewater. (Use part (iv) and (v) for this question).
Answer: Mass = Moles x Mr
Mass = (0.0234) x (74) = 1.73 g
With this, our moles related question has come to an end. If you are having trouble understanding any question, do leave a comment below.
Some other ATP Concepts:
- The presence of an impurity in a compound will lower its melting point (because it causes the forces between molecules to easily overcome).
Moreover, the impurities (in a solution) increase the boiling point. This is because more heat is required to vaporize the impure solution.
- The table below shows the colour changes of some indicators (these are often asked in the moles related questions).
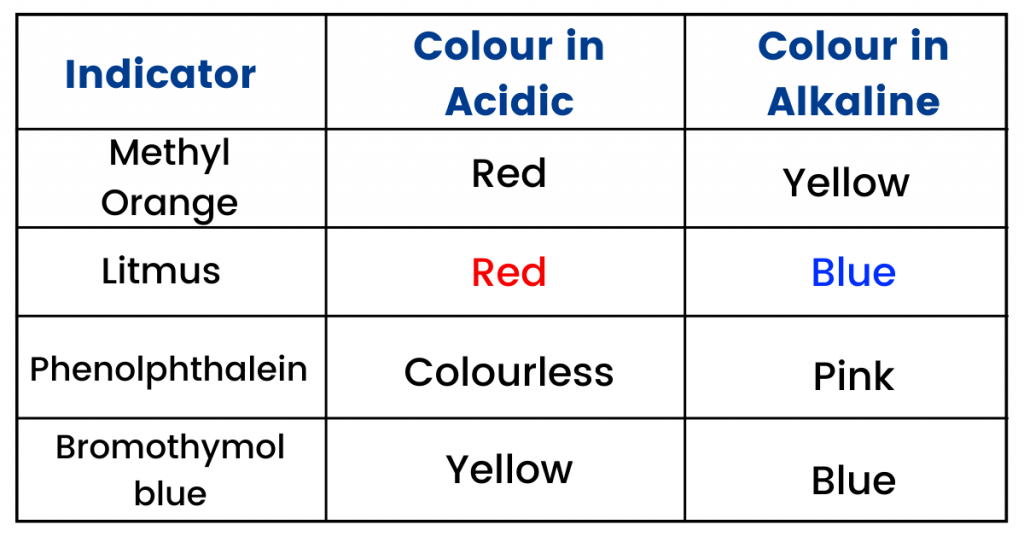
- To ensure that the results are accurate (and reliable), we repeat the experiment and takes the average values.
Conclusion:
Now, I turn it over (to you people).
Which alternative to the practical (ATP) concept you enjoyed reading the most? And which topic you found the most challenging?
Anyway, do let me know in the comments below.
Some important topics, such as the identification of cations and anions and the colours of indicators have been discussed in detail. Thank you very much for reading and staying with me till the end. Stay tuned for more.

Excellent points!🙌🏻
Glad about that!