If you want to learn, how to calculate the molar mass in periodic table, you will love this step-by-step explanation.
But first of all, let’s start with a simple introduction.
In chemistry, the molar mass of a compound (represented by M = m / n) is the mass of the substance (in grams), divided by the number of moles of the substance.
Let me make it simple for you.
Definition of Molar Mass:
Let’s suppose you have a scale of 12 units. Now, the carbon-12 isotope has a mass of exactly 12 units. So the relative mass of one molecule of a compound is said to be the molar mass of that compound.
Note: Do you know the difference between molar mass and relative molecular mass? Molar mass is the mass of Avogadro’s number of molecules (6.02 × 1023).
You can also say that it is the mass of one mole of a substance and is represented in grams (g/mol to be exact).
Molecular mass (also known as molecular weight) is also the mass of one molecule of a substance. It is represented in amu/u. However, the numerical values of both of them are the same.
For example, the molecular mass of 1 H2O molecule is 18 a.m.u. Similarly, the mass of 1 mole of H2O molecule is 18g.
Pretty simple, isn’t it?
But before telling you how to calculate the molar mass using the periodic table, let me tell you what a periodic table is.
Periodic Table:
The periodic table of chemical elements is a table that displays the chemical elements. As you may know, these elements are arranged by (increasing) atomic number (number of protons in the nucleus of the atom).
With this, it is time to move on and talk about the calculation of molar mass.
How to find the molar mass on the periodic table?
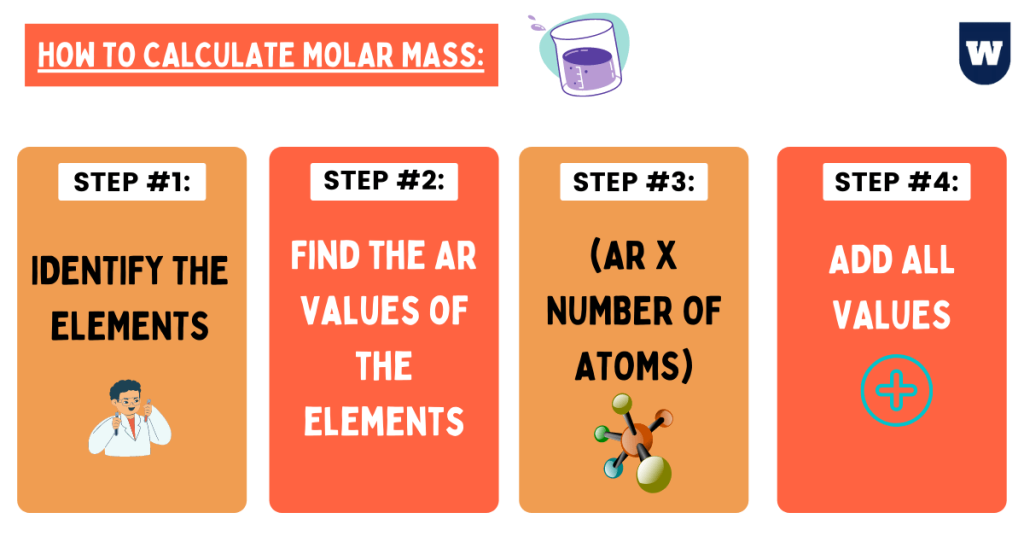
To find the molar mass, we are going to do four simple steps:
- Identify the element(s)
- Use the periodic table to find the A r value(s) of the element
- Multiply the number of atoms with the Ar value
- Add the values (if possible)
Let me explain all this to you with the help of some examples:
The molar mass of compounds:
- Molar mass of sulfuric acid (H₂SO₄):
Recall the three steps mentioned above.
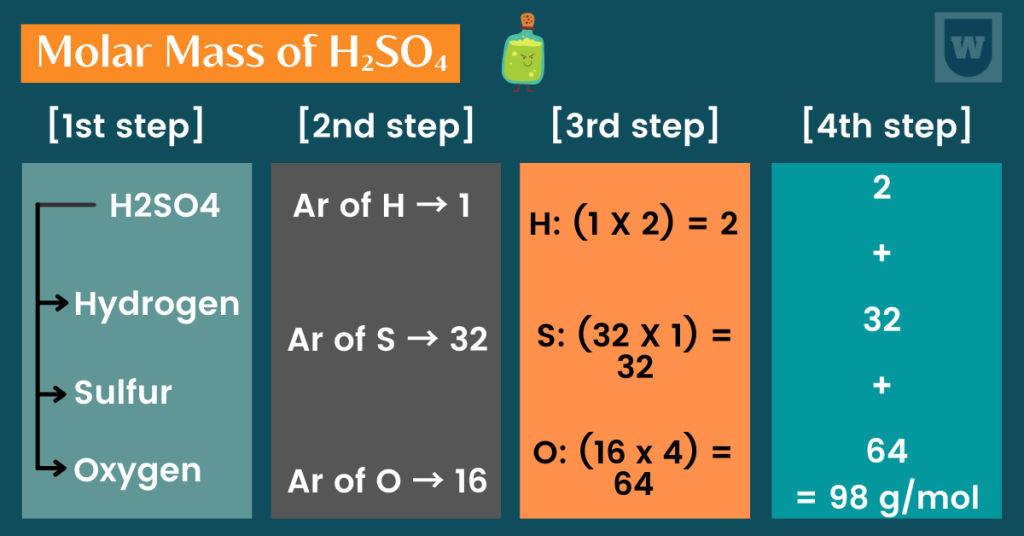
[1st step]: First of all, we will identify all the elements in this compound. From the formula H2SO4, we can say that this compound has three elements:
Hydrogen, sulfur and oxygen.
[2nd step]: Now, let’s use the periodic table to find the Ar values of these elements.
Hydrogen → 1
Sulfur → 32
Oxygen → 16
When you have used the periodic table to calculate the Ar values, move on to the third step.
[3rd step]: From the formula (H2SO4), we can say the following:
Hydrogen has two atoms (suggested by H2). Moreover, sulfur has only one atom (shown by S), and oxygen has four atoms (shown by O4).
So now, we are going to multiply the number of atoms with their Ar values that we found out in step 2. In simple words, we are going to do this: (Ar of the element x number of atoms of the element).
For hydrogen: (1 x 2) = 2
For Sulfur: (32 x 1) =32
For Oxygen: (16 x 4) = 64
[4th step]: Finally, you are going to add all the values to get your molar mass. In this case, it will be:
2 + 32 + 64 = 98 g/mol
Now, let’s take a look at some other examples as well.
- The molar mass of silver nitrate (AgNO3)
[1st step]: From the formula, can you find out what elements are present in silver nitrate?
We have silver (Ag), nitrogen (N) and oxygen (O). With this, it is time to move on to the second step.
[2nd step]: Here are the Ar values of these elements:
Silver → 107
Nitrogen → 14
Oxygen → 16
[3rd step]: Now, we are going to use this formula: (Ar of the element x number of atoms of the element).
For silver: (107 x 1) = 107
For nitrogen: (14 x 1) = 14
For oxygen: (16 x 3) = 48
[4th step]: Now, we are going to add these values to get the molar mass of silver nitrate (AgNO3)
107 + 14 + 48 = 169 g/mol
Here is another example for you.
- The molar mass of Aluminium oxide (Al2O3)
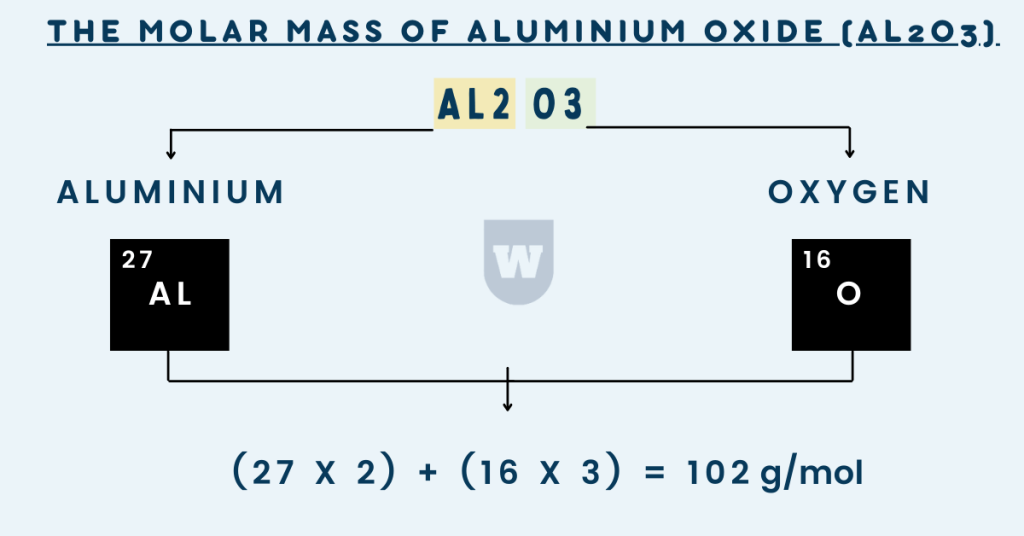
[1st step]: What elements are present in aluminium oxide? After looking at the formula, you will say:
Aluminium and oxygen. When we know this, it is time to move on to the second step.
[2nd step]: Here are the Ar values of these two elements:
Aluminium → 27
Oxygen → 16
[3rd step]: Now, we are going to use this formula: (Ar of the element x number of atoms of the element).
For Al: (27 x 2) = 54
For O: (16 x 3) = 48
[4th step]: Now, the molar mass would be:
54 g/mol + 48 g/mol = 102 g/mol
Summary: To find the molar mass in periodic table, we do four steps. First of all, we identify the elements in the compound. For example, the elements in AgCl are silver and chlorine.
Then, we find the Ar values of the elements. Then, we apply the formula: (Ar of the element x number of atoms of the element). Finally, we add all the values to get the molar mass in g/mol.
How to convert molar mass to moles?
When studying this topic, it is important to learn this calculation. But first of all, let me introduce moles to you.
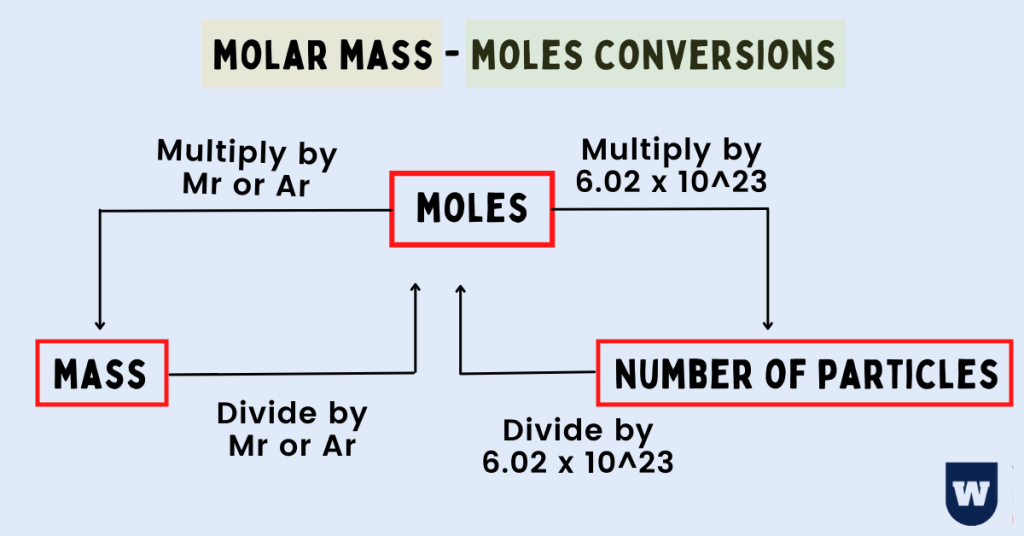
Mole: A mole is a mass of a substance that consists of 6.023 x 1023 particles of the substance. In simple words, it is the SI unit (International System of Units) for the amount of substance.
To convert molar mass to moles for a substance, we divide the mass of the substance by its molar mass. This is represented by this formula:
Moles = (Mass of the substance) / (Molar mass)
Further Reading:
Covalent Bonding of water (H2O) | Must Read
Ionic bonding of NaCl | Sodium Chloride
Electrolysis Made Simple | Best Guide
Here is a simple example.
Q: The molar mass of sodium hydroxide (NaOH) is 40 g/mol. How many moles are present in 90 g of NaOH?
Solution:
Recall the formula for moles:
The number of moles = Mass / Molar mass
Moles = 90 / 40 = 2.25 moles
So this is how you can use mass and molar mass to calculate moles. But, how can you calculate the molar mass using moles?
To do so, we are simply going to rearrange the above formula:
Molar mass = Mass / Moles
Here is an example.
Q: What is the molar mass of CH4 (methane) if there are 0.623 moles in a 10 g sample?
Here is the solution:
The formula is: Molar mass = mass / moles
Molar mass = 10 / 0.623
= 16.05 g/mol
So this was the simple concept about these conversions.
Molar Mass – Complicated problems:
When you are given the formula, things become much easier. Agree?
But, what if you have to deduce the formula first before calculating the molar mass? Here is what I mean.
The question says: What is the molar mass of calcium phosphate?
The first thing to note over here is that calcium phosphate is an ionic compound (a compound formed between a positive and a negative ion).
Now, here is the exact process that you will use to find the formula of ionic compounds:
- Identify the ions (cations and anions)

In this case, we have two ions. The cation (positive ion) is calcium, and the anion (negative ion) is phosphate.
The calcium ion has a 2+ charge. This is because it is in the second group of the periodic table (the group number shows the number of charges on the ions).
Calcium ion → Ca2+
Moving onto the anion. The anion, Phosphate (PO4), has a charge of 3-. Here’s how.
The valency of O is 2 (as it needs two more electrons to complete its outer shell). Since there are 4 oxygen atoms, we can say: (2 x 4) = 8
Moving on, the valency of P is 5. So the net valency of the phosphate ion would be: (8 – 5) = 3
Phosphate ion → PO₄³⁻
This takes us straight to the next step.
- Write the ions separately
This process is simple. From the first step (where you have identified the ions), just simply write down the ions in front of each other. (Look at the image above for reference).
Ca2+ PO₄³⁻
- Cross multiply their powers
Now simply, cross multiply the powers to get the final formula. In simple words, exchange the powers of both ions.
So the formula of calcium phosphate would be: Ca3(PO4)2
Now, you can simply calculate the molar mass of this compound.
The molar mass of calcium phosphate: (40 x 3) + (31 x 2) + (16 x 8) = 310 g/mol
Now, here is another question for you.
Q: What is the molar mass of Vanadium (V) Hydrogen Phosphate?
First of all, let’s identify the ions.
This compound is made up of vanadium (V) cations and hydrogen phosphate anions. Here is what I mean:
Vanadium (V) cation → V5+
Hydrogen Phosphate anion → HPO42-
Note: The hydrogen phosphate anion is derived like this: H+ + PO43- → HPO42-
Now, let’s write down the ions separately and cross multiply (exchange) the powers.
V5+ HPO42-
So, the formula for vanadium (V) hydrogen phosphate will be: V2(HPO4)5
Now, we can calculate the molar mass of this compound (using the steps I mentioned above).
Ar of Vanadium → 50.9
Ar of Hydrogen → 1
Ar of Phosphorus → 30.9
Ar of Oxygen → 15.9
Molar mass: (50.9 x 2) + (1 x 5) + (30.9 x 5) + (15.9 x 20) = 579.3 g/mol
Wrapping Up:
With this, our topic about molar mass in the periodic table has come to an end. I shared the exact step-by-step strategy that you can use to calculate the molar mass of any compound.
Now I turn it over to you.
Which part of this topic do you find the most interesting? Do let me know in the comments below.
Just to summarise, the molar mass is the mass of the instance divided by the number of moles of the substance. Thank you for reading. Stay tuned for more.
