If you want to know about the ionic bonding of sodium chloride (NaCl), you will love this comprehensive resource I am about to share with you.
So if you want to understand this concept, this simple explanation will help you do so.
Keep reading.
What is Ionic Bonding:
Ionic bonding (a branch of chemical bonding) refers to the formation of ionic bonds (that form between two or more atoms due to electron transfer).
Now you might be wondering, how do these bonds form? We will take a look at that in detail. But before that, let’s discuss what is meant by cations and anions.
In simple words, cations are positive ions. And they are formed when an atom loses electrons (such as metals).
On the other hand, anions are negative ions, and they are formed when an atom gains electrons (such as non-metals).
Simple, isn’t it?
In short, here is what you need to know.
An ionic bond is a type of linkage formed between oppositely charged ions (in a chemical compound) due to their electrostatic attraction.
How do Ionic bonds form?
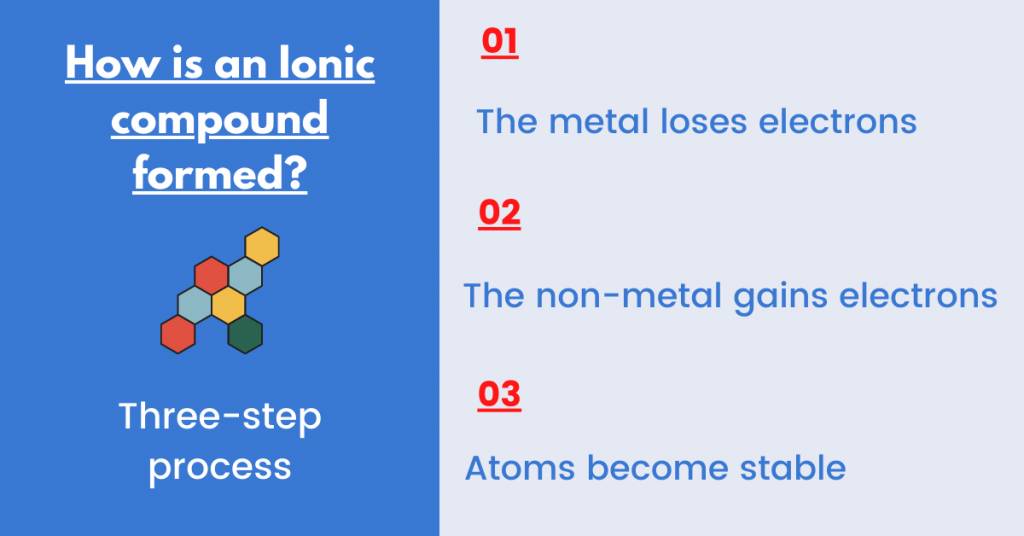
As I said earlier, ionic bonds form when an exchange of electrons takes place (the loss and gain of electrons).
But, let’s dive deep into this topic.
Here is the reason why this transfer of electrons takes place:
- It allows an atom to achieve an electronic configuration of a noble gas.
But what does it mean? Let me explain
We say that noble gases are stable (inert). This means that they do not need to gain or lose electrons. Therefore, ionic bonding allows the atom to have fully filled valence (outer) shells.
Note: If an atom has a completely filled outer shell, it means that it is stable.
In other words, atoms become stable due to this movement of electrons.
At this stage, you should be also familiar with duplet and octet electronic configuration.
Duplet and Octet electronic configuration:
In Chemistry, the duplet or octet rule allows an atom to complete its number of electrons (become stable). Here is what I mean.
The difference between duplet and octet rules is that a duplet is an atom or ion having a maximum of two electrons in its outermost (valence) shell.
However, an octet is an atom or ion having a maximum of eight electrons in its outermost shell.
With this, it is time to move on and talk about the ionic bonding of sodium chloride (NaCl).
Ionic Bonding of Sodium Chloride (NaCl).
Sodium chloride (widely known as salt) is an ionic compound and has the chemical formula NaCl.
Below is the complete procedure on how sodium chloride is formed.
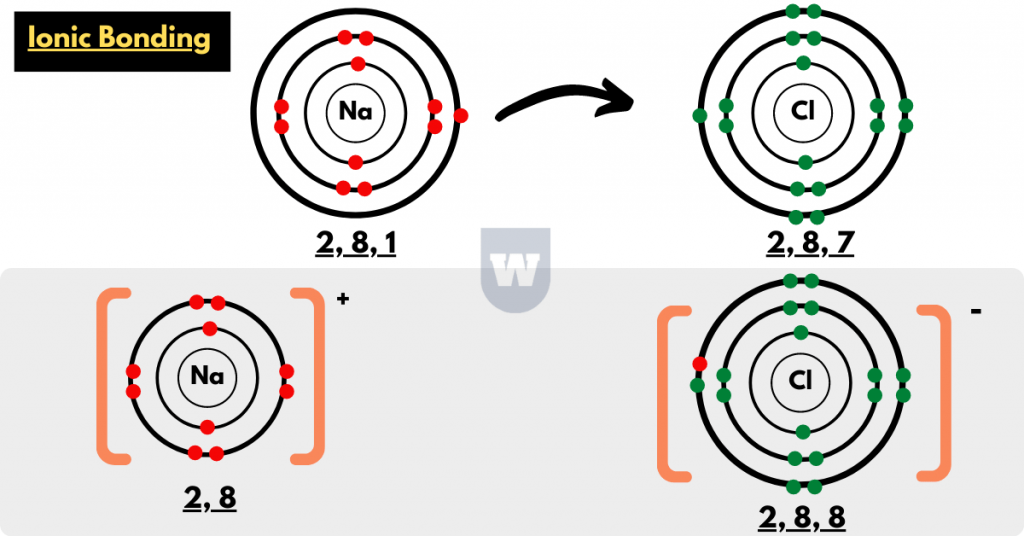
- Sodium (a soft, silvery-white highly reactive metal) has an atomic number of 11.
This means that a Sodium atom has 11 protons and 11 electrons. Thus, the electronic configuration of this atom is:
2, 8, 1
- Chlorine (a chemical element with symbol Cl) has an atomic number of 17.
This means that the Chlorine atom has 17 protons and 17 electrons. Therefore, the electronic configuration of a Chlorine atom can be written as:
2, 8, 7
- Now, closely observe the electronic configuration of both the atoms.
You will notice that the Sodium atom needs to lose 1 electron to become stable (achieve octet configuration).
But, the Chlorine atom needs to gain 1 electron to become stable (achieve octet electronic configuration).
The image below will help you understand all this.
With this, it is time to discuss how to write the formula of an ionic compound.
Further reading:
Exothermic and Endothermic reactions | Energy changes Notes
To derive the formula, we will do three steps:
- Identify the cation (write its state symbol and its valency).
For example in Sodium Chloride, the cation is Na and has a valency of +1 (or simply +).
2. Then, identify the anion (note the state symbol and the valency).
For example in Sodium Chloride, Cl is the anion and has a valency of -1 (or simply -).
3. Finally, cross multiply the valencies to obtain the formula. This is only known as the “balancing of charges“.
Here is an example for you to further understand this concept. In this example, we are going to find out the formula of Aluminium Oxide.
The cation is Aluminium (Al) and has a charge of +3. The anion is Oxide (O) and has a charge of -2.
So from all this, the formula of Aluminium Oxide (after balancing the valencies) would be:
Al₂O₃
Summary: In the ionic bonding of Sodium Chloride (NaCl), the sodium atom loses one electron (to become a cation), and the chlorine atom gains one electron (to become an anion). In this way, a positive sodium ion (Na+) and a negative chloride ion (Cl-) form an ionic bond.
Moving on, let’s take a look at some characteristics of ionic bonding.
Charactersticks of Ionic Bonding:
Sodium Chloride is an ionic compound. But, what are some characteristics (properties) of compounds formed by ionic bonding?
Let’s discuss.
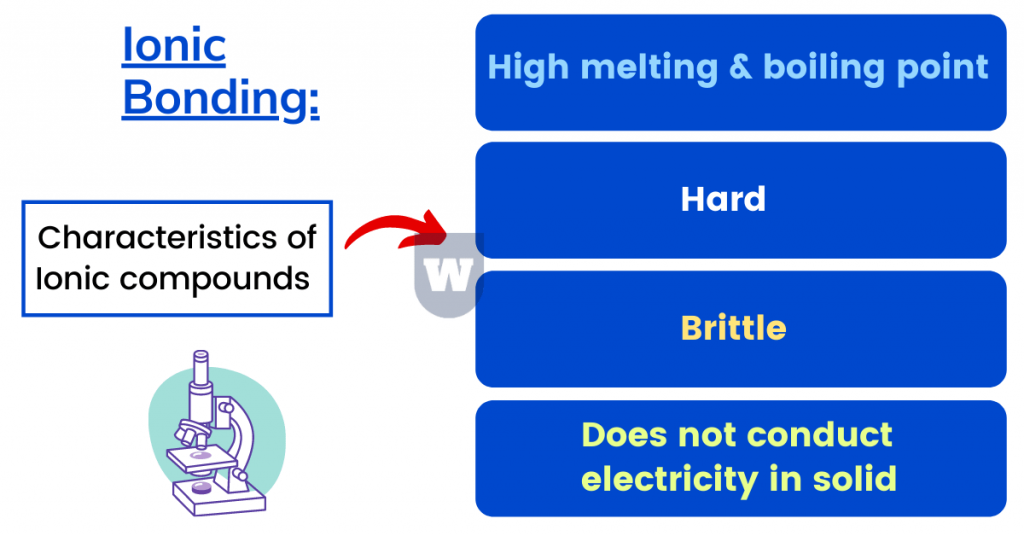
- Ionic compounds have high melting and boiling point.
Do you know that the melting point of Sodium Chloride (NaCl) is 801°C and its boiling point is 1413°C?
Pretty high, isn’t it?
Here is the reason for that.
Recall your knowledge about ionic bonding. We learned that ionic compounds have strong electrostatic forces of attraction between oppositely charged (positive and negative) ions.
Therefore, a very high temperature is required to overcome these strong forces of attraction.
You should also remember that ionic compounds have a giant lattice structure. Thus, a large amount of heat energy is required to break this structure.
- Ionic compounds are brittle but hard. Here’s why.
You should know that it takes a large amount of (mechanical) force to allow ions to slide over each other. However, when it is done, ions of the same charge get close to each other.
As we know that like charge repel, so a force of repulsion is produced over here (which causes them to shatter).
In short, the strong repulsive forces shatter the compound (crystals).
- Ionic compounds conduct electricity in molten or aqueous solutions.
Do you know that ionic compounds in the solid-state do not conduct electricity? The reason for it is that ionic compounds (in this case) have no free-moving (mobile) electrons or ions to conduct electricity.
In an aqueous solution, ionic compounds have free moving ions that allow them to conduct electricity. In the same way, molten (melted) compounds will also conduct electricity due to the presence of electrons.
Now, it is time to test your knowledge. Below are some questions for you (the answers are given at the end of all the questions).
Ionic bonding questions:
Question 1: How do metal atoms form ions?
A. They gain electrons to form positive ions.
B. They lose electrons to become positive ions.
C. They lose electrons to form negative ions.
Question 2: Which of the following is not an ionic compound?
A. Sodium Chloride (NaCl)
B. Lithium Bromide (LiBr)
C. Water (H2O)
Question 3: Which statement is not true about ionic bonds?
A. They have a three-dimensional structure known as the lattice structure.
B. In a solid state, they do not conduct electricity.
C. Ionic bonds are formed between non-metals.
Question 4: Why is the melting point of Magnesium Oxide higher than Sodium Chloride?
A. Magnesium Oxide is a covalent compound
B. Magnesium and Oxide ions have a greater number of charges than Sodium and Chloride ions
C. Sodium Chloride has a giant lattice structure while Magnesium Oxide does not.
Question 5: What is true about comparing the electronegativity of Sodium and Fluorine?
A. If compared to Sodium, Fluorine has a high electronegativity. So Fluroine attracts an electron.
B. If compared to Sodium, Fluorine has a high electronegativity. So Fluroine loses an electron.
C. If compared to Fluorine, Sodium has a high electronegativity. So Sodium gains an electron.
Answers:
B
C
C
B
A
Ionic Bonding: Frequently Asked Questions
What are 5 examples of ionic bonds?
Sodium Fluoride (NaF), Lithium Bromide (LiBr), Sodium Iodide (NaI), Lithium Chloride (LiCl) and Magnesium Chloride (MgCl2) are some examples.
Note how they are formed between a metal and a non-metal.
How do you determine ionic bonding?
To find out whether a bond is ionic or covalent, you can use the following:
1. A compound made from a metal AND non-metal will have ionic bonds.
2. A compound made from two non-metals will have covalent bonds.
Conclusion:
Now, it is your turn.
Which part of this topic did you find the most interesting. Was it about the ionic bonding of Sodium Chloride (NaCl)? Or it was about the characteristics of ionic bonds?
Is there (still) any concept you find challenging?
Either way, do let me know in the comments below. Just to recap, here are some of the (prominent) topics we covered:
What is meant by ionic bonding, and how are ionic bonds formed?
Thank you very much for reading and staying with me till the end. Stay tuned for more.
