If you want a step-by-step guide on thermal physics, you will love this comprehensive resource I am about to share with you.
First of all, let me tell you that thermal Physics is the study of heat and temperature. In other words, it is the combined study of the kinetic theory of gases and thermodynamics.
Now when you know something about this topic, let’s dive into some concepts without further introduction.
Thermal Physics
Kinetic Particle Model of Matter:
The first concept that you should know is pretty simple.
But first, I have a question for you. How do you differentiate between a solid, liquid and gas?
Here is the basic difference that you should know.
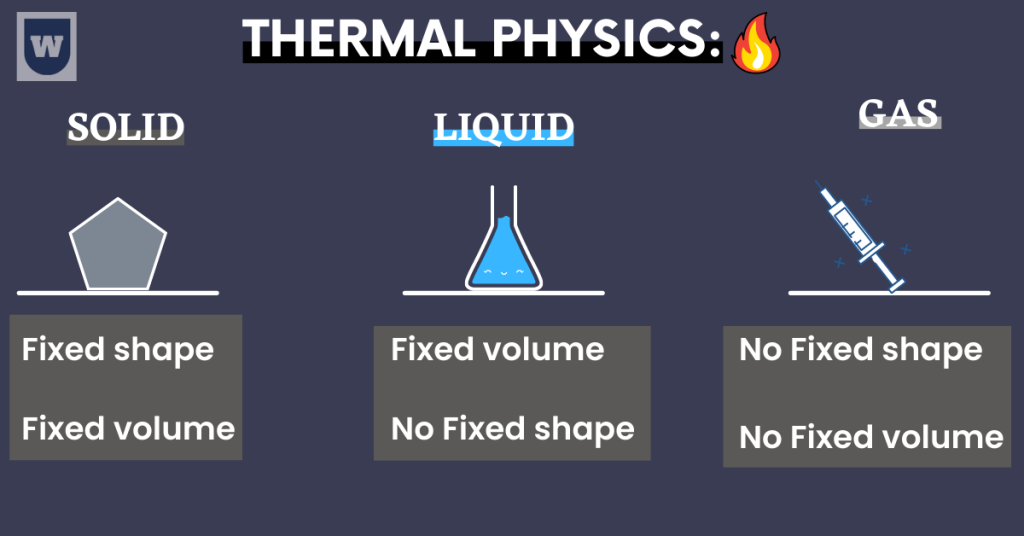
- Solids: They have a definite volume and shape. Thus, they are relatively rigid.
This simply means that the atoms and molecules in a solid are attracted to each other. Therefore, they ONLY vibrate and cannot move around.
- Liquids: They have a definite volume because the particles are in close contact. But here is something fun.
They DO NOT have a definite shape. This is because the particles in a liquid can move freely. Moreover, the particles are bound by weak intra-molecular forces.
In short, the particles move around but they still remain close to each other.
Pretty simple, isn’t it?
- Gases: The concept over here is simple. They do not have a definite shape and volume. Here’s why.
You should know that the particles in a gas are spread far away from each other. And the particles are moving freely. As a result, the gases are loosely packed and they have large intermolecular spaces.
So gases can acquire any volume or shape.
Now when you know the basics, here is an important concept that you should know.
Kinetic molecular theory of matter:
This particle theory says that all matter has many small particles that are in constant and random motion. Plus, the degree to which they move is determined by their energy.
Note: You can be asked to write the above definition as well.
With this, it is time to move on and talk about the particle model.
The Particle Model:
I have a question for you. What is the relation between the motion of particles and temperature?
Before understanding this concept about heat and temperature, recall that matter consists of moving particles (atoms or molecules). And these particles interact with one another.
So here is what you need to know.
- If we increase the temperature, the motion of particles is also increased. This is because particles start to vibrate faster. As a result, their Kinetic energy increases.
On the other hand, if the temperature is lowered, the motion of particles is also reduced.
Over here, you should also know about the term “absolute zero“.
Absolute Zero:
To measure temperatures for scientific work, we use a scale known as the Kelvin scale. You should know that this scale shows a temperature of 273.16 ºC for melting ice.
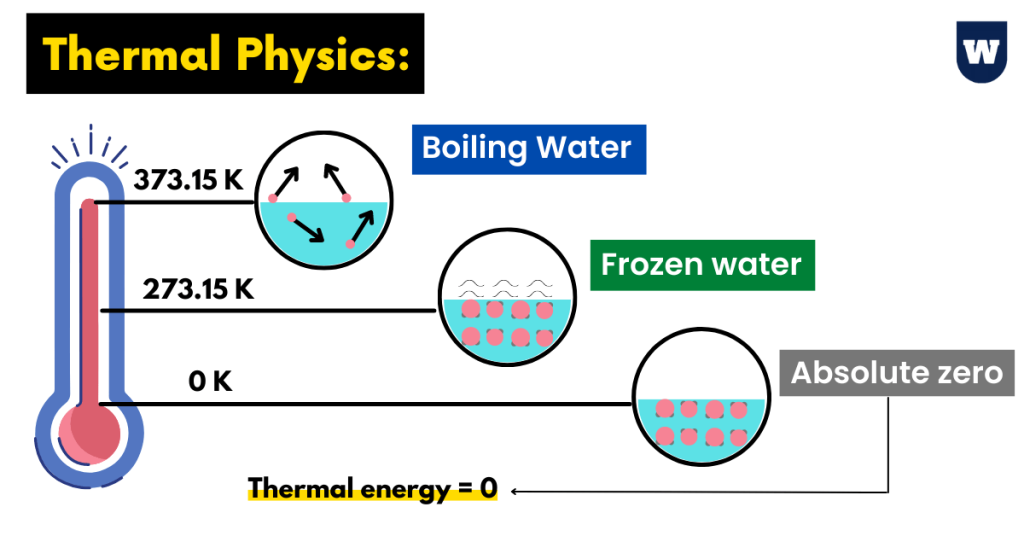
So to convert celsius temperature to Kelvin temperatures, we simply add 273.16.
K = C + 273
But why is this important?
Note that the temperature of 0.0 K is known as absolute zero. And this is the lowest temperature that can be obtained.
In other words, it is the temperature at which a system (thermodynamic) has the LOWEST energy. At this temperature, the particles in a substance are motionless.
You can also say that at this stage, particles have the least kinetic energy.
With this, it is time to move on to the next concept.
The Gas Laws:
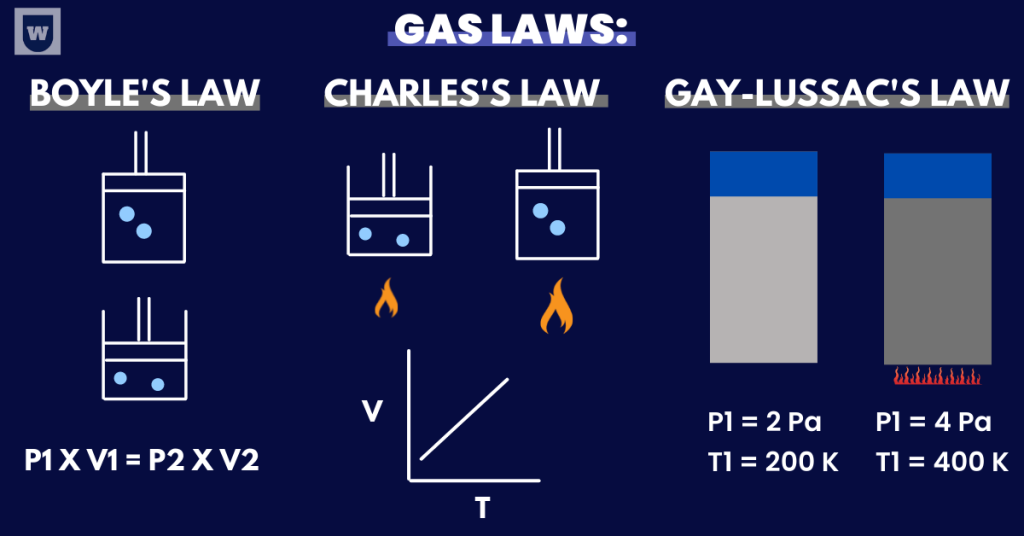
- The relationship between pressure and temperature at constant volume.
You should know that the pressure of a gas is directly proportional to the temperature if the volume is kept constant.
This means if the pressure increases, the temperature also increases, and vice versa. Do you know why?
Recall that increasing the temperature also increases the kinetic energy of particles. This causes them to move faster and collide more frequently with the walls of the container.
As a result, the pressure of the system also increases. This is known as the Gay-Lussac’s Law.
Pretty simple, isn’t it?
- The relationship between volume and temperature at constant pressure.
According to Charles’s Law, the volume of a gas is directly proportional to the (absolute) temperature at a constant pressure.
Here is what I mean.
When the temperature increases, the particles gain kinetic energy and start to move faster. As a result, they overcome the intramolecular forces of attraction and move further away.
This causes the volume of the system to increase.
- The relationship between pressure and volume at a constant temperature.
According to Boyle’s Law, when the pressure of a gas increases, the volume decreases. This means that these quantities are inversely proportional to each other.
Do you know why? Here is a simple explanation.
The decrease in volume means that the same amount of gas particles come close together. In other words, the gas is compressed so it takes less space.
As a result, the particles collide more frequently and the pressure increases.
Pretty simple, isn’t it?
To solve questions regarding this concept, you should learn this expression:
(P1) x (V1) = (P2) x (V2)
where P1 and V1 are the initial volume and pressure values.
where P2 and V2 are the final volume and pressure values (after change).
This takes us straight to the next topic, thermal properties and temperature.
Thermal properties and temperature:
Measuring Temperature:
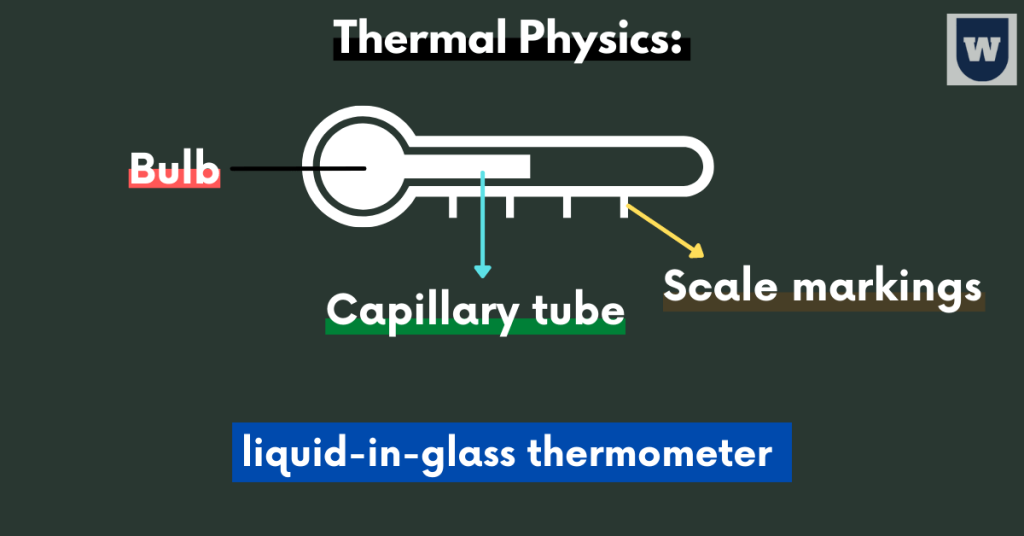
First of all, let me introduce the liquid-in-glass thermometer to you.
The liquid-in-glass thermometer contains a liquid in a thin capillary tube that expands with temperature. Plus, we have a glass bulb at the end of the tube.
You should know that this glass bulb contains a larger volume of liquid, which moves in the narrow tube when heated.
Let me explain all this to you in detail. But first of all, let’s talk about “fixed points“.
When we calibrate a thermometer, we identify two points at which an (identifiable) change occurs. For example, the boiling of pure water at 100ºC and the melting of ice at 0ºC.
In simple words, we identify an upper and a lower point on a thermometer. And in a celsius scale, we have the following fixed points:
- The Ice point: It refers to the temperature of melting ice (0ºC) and is used as a lower fixed point.
- The steam point: It refers to the temperature of the steam from water boiling (100ºC). And it is used as the upper fixed point.
Before moving on to the next topic, here are some important terms that you should know.
- Sensitivity: It is a property of a thermometer that helps it to detect minor (small) temperature changes.
In other words, sensitivity is the increase in length of the mercury column per unit increase in temperature.
- Range: It is the difference between the maximum and minimum temperature that a thermometer can read.
Let me make this simple for you.
Let’s say that –10ºC is the lowest temperature that a liquid-in-glass thermometer can read. And 110ºC is the maximum temperature that the thermometer can show.
So the range will be –10°C to 110°C.
Simple, isn’t it?
Factors affecting the sensitivity of liquid thermometers:
This is one of the MOST important concepts in this topic. In fact, this concept is tested frequently in the MCQ’s (multiple choice questions) paper.
So here is what you need to know.
Below are some factors that affect the sensitivity of a liquid-in-glass thermometer:
- Using a narrow bore (tube): This increases the sensitivity of liquid thermometers. Can you guess why?
This is because heat is easily transferred to the bulb. So a small change in the volume causes the liquid to move a larger distance.
In other words, a narrower bore has less surface area which allows it to show greater and faster results.
- Using a thermometer with a smaller glass bulb: This also increases the sensitivity of a thermometer. Here’s why.
If you have a smaller bulb, this means that there is less “thermal mass“. This takes less time to warm up. Therefore, a smaller bulb reacts faster to changes in temperature.
In simple words, heat is absorbed in a shorter time by the “less liquid”.
Further reading:
Light Made Simple | O Level Physics | Best Notes
Magnetism and electromagnetism | Detailed Notes
Current Electricity | The Ultimate Guide | Notes
Simple, isn’t it?
Note: Using a glass bulb with a thinner wall also increases the sensitivity of a thermometer. This is because heat is easily transferred to the bulb.
With this, it is time to move on and talk about the thermocouple thermometer.
Thermocouple thermometer:
What do you understand when I say the term “thermocouple”?
A thermocouple is a temperature-measuring device that consists of two wires of different metals joined at each end.
But the question is, how can we use thermocouples to find the temperature?
Firstly, we have two “junctions”:
- “Measuring junction” or “hot junction“.
- “Reference junction” or “cold junction“.
The concept over here is that when the temperature rises or falls at the hot junction, a voltage is generated. This voltage (e.m.f) is detected by a measuring device connected at one end.
We then use this data to find out the temperature using standard tables. This simply means that we compare the voltage and temperature values.
Note: Copper and Iron are metals that can be used in a thermocouple thermometer. And, the e.m.f produced is directly proportional to the temperature difference.
Specific heat capacity:
I have a question for you regarding thermal physics. What is internal energy?
Internal energy is the total energy of all the particles in a substance. But, why is it important?
The concept of internal energy will help us understand topics such as the change of state. And if we increase the temperature, the internal energy of an object also increases.
Now, let’s talk about specific heat capacity.
According to Cambridge, specific heat capacity is the energy required per unit mass per unit temperature increase.
In other words, it is the amount of thermal energy required to raise the temperature by 1K or 1°C of a unit mass (1kg).
Here is the formula to calculate specific heat capacity (c):
specific heat capacity (c) = [ change in energy (∆E) ] / [ mass x temperature (∆θ) ]
You may also re-arrange this formula as ∆E = mc(∆θ). And the SI unit is J kg-1 K-1.
With this, it is time to move on to the next topic.
Melting, boiling and evaporation:
You should know that during a change in state (from solid to liquid or liquid to gas), the temperature remains constant.
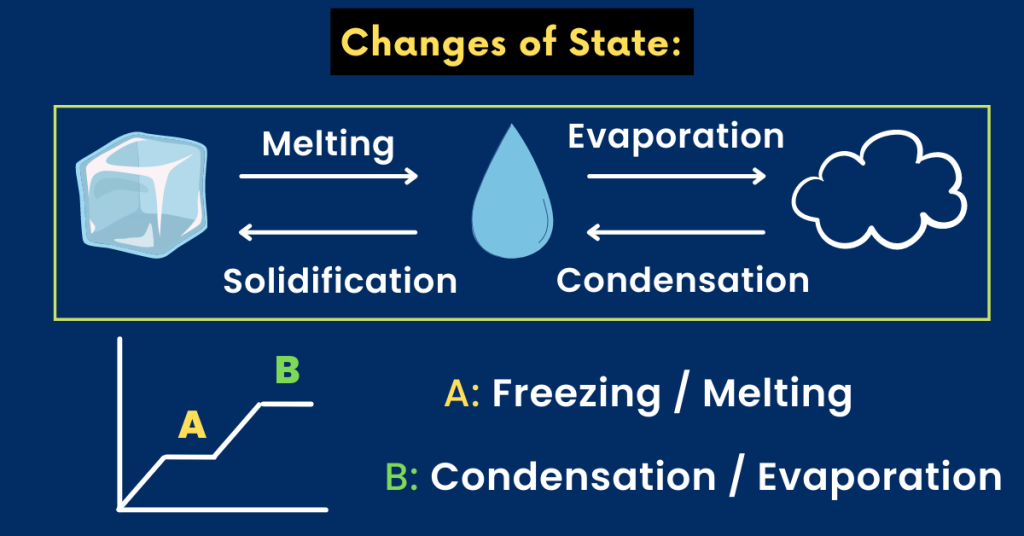
This is because all the heat energy released or gained is used to form or break the bonds. Therefore, the temperature DOES NOT increase or decreases during this phase.
Here are some definitions that you should know about:
- Melting: Do you know that melting is a process in which energy is required to break the cohesive bonds in a solid?
Let me make this simple for you.
Melting is a process in which a solid changes to a liquid. For example, when the ice melts it changes into water. So here is what you need to know.
The heat from the air transfers to the ice which causes it to melt. This energy (called heat of melting or heat of fusion) is absorbed as potential energy by the particles. As a result, the solid changes to a liquid.
Now let’s talk about solidification.
- Solidification: In this process, atoms are converted from a liquid disordered state to a solid ordered state.
Let me explain.
Solidification is a process in which a molten liquid changes to a solid. An example is the freezing of water to form ice.
Pretty simple, isn’t it?
- Condensation: It is the process by which water vapours (gas) change into liquid water. Do you know why is this important?
This process plays an important role in the water cycle during the formation of clouds.
This process can occur in two ways:
When the air is cooled to its due point (the temperature at which condensation happens).
OR
When the air becomes completely saturated with water vapours, it cannot hold more water. Now, let me introduce boiling and evaporation to you.
The difference between boiling and evaporation:
This is an important concept as it is frequently tested in exams. So here’s what you need to know.
In boiling, a liquid evaporates when heated to its boiling point. In simple words, a liquid is converted into gas upon heating.
During boiling, the vapour pressure of the liquid overcomes the atmospheric pressure. As a result, bubbles are formed and they rise upwards.
Now, let’s talk about evaporation.
Evaporation is a process in which a liquid changes to a gas at a temperature below its boiling point.
This is because the MORE energetic molecules escape from the liquid. This explains why evaporation causes cooling. Here are some factors that affect evaporation:
evaporation rate ∝ Surface Area (directly proportional)
Rate of Evaporation ∝ Wind Speed (directly proportional)
Rate of Evaporation ∝ Temperature (directly proportional)
Rate of Evaporation ∝ 1∕ Humidity (inversely proportional)
This simple table below summarises the difference between evaporation and boiling.
| Evaporation: | Boiling: |
| 1. It can occur at any temperature. | 1. It occurs at a particular temperature. |
| 2. Takes place at the liquid surface. | 2. Takes place throughout the liquid. |
| 3. Temperature may change. | 3. Temperature remains constant. |
| 4. Thermal energy source is NOT required. | 4. Thermal energy source is required. |
With this, it is time to move on to the next topic.
Transfer of thermal energy:
Do you know that there are three ways in which thermal energy can be transferred? These are:
- Conduction
- Convection
- Radiation
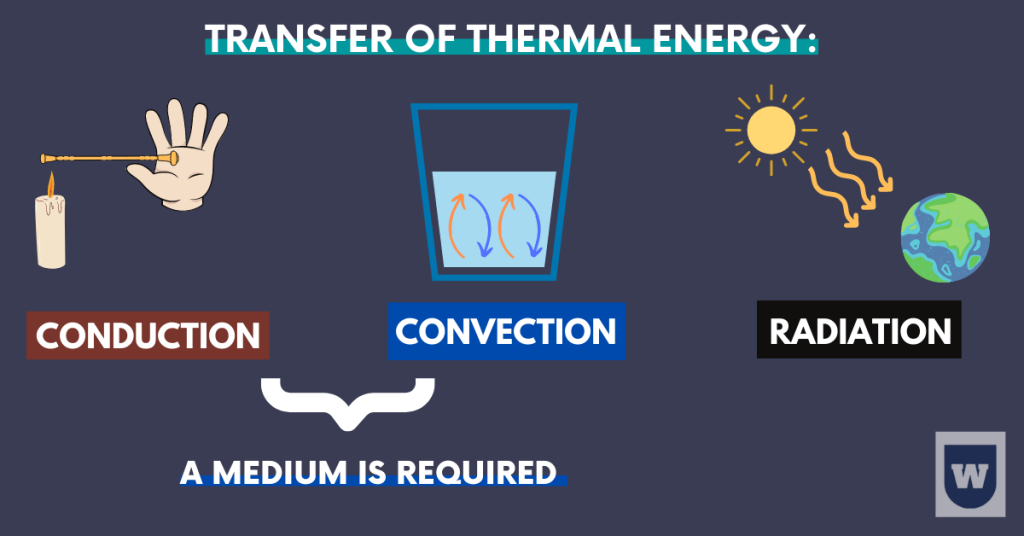
Let’s talk about each of them in detail.
Conduction:
It is a process that occurs through the interaction of neighbouring molecules and atoms, transferring heat energy to their neighbouring particles.
In other words, this process allows heat transfer to occur in solids. Let me explain.
Note: Remember that heat transfers from a region of higher temperature to a region of lower temperature.
Here’s how conduction takes place.
- A solid is heated up.
- The heat is gained by the vibrating atoms (near the source). The heat energises the atoms that cause vibrations to increase.
- These vibrations spread throughout the solid. As a result, the entire solid is heated.
Over here, you should know that this is a slow process. Now, let’s talk about convection.
Convection:
This process occurs in fluids (liquids and gases).
But how does this process occur? Let me explain how convection takes place.
When a fluid is heated from below, it expands (thermal expansion). The lower layer of the fluid becomes less dense.
Now, you know that the colder layer of fluid above is denser. So due to buoyancy, the hotter (less dense) layer of fluid rises up. And the colder (denser) layer of fluid sinks.
Some examples of convection are:
- Land breeze
- Sea breeze
Now let’s talk about radiation.
Radiation:
Do you know that heat can be transferred by infrared radiation? And during this heat transfer, particles are NOT required.
Instead, this method of heat transfer involves waves. Let me explain.
But first, you should know that some surfaces are better at reflecting and absorbing infrared radiation. Here are the details.
Shiny → Poor absorber
Shiny → Poor emitter (but good reflector)
Dull, matt or rough → Good absorber
Dull, matt or rough → Good emitter
Importance of thermal energy transfer:
To better understand radiation, let’s take a look at some applications of radiation.
In a vacuum flask, we have a double-walled glass container with silvered surfaces. Do you know why these walls of the glass are silver?
This is because these silvered walls reflect infrared radiations back into the hot liquid. As a result, heat transfer through radiation is minimized in a vacuum flask.
Pretty simple, isn’t it?
Wrapping Up:
With this, this detailed guide on thermal physics has come to an end. Note that this guide fulfils the syllabus requirements for the 2022 syllabus. Plus, the requirements for the 2023-2025 syllabus have also been met.
You should practise plenty of past papers because this is an important topic from an examination point of view.
Now I turn it over to you. Which concept of this topic do you find challenging? Is it related to thermometers? Or is it is related to the transfer of thermal energy?
Either way, do let me know.
Thank you for reading and staying with me till the end. Stay tuned for more.
