Do you know that intermolecular forces (IMF) are the forces faced by atoms, ions and molecules (neighbouring particles) when they are placed close to each other?
And if you want to know about intermolecular forces in HF (Hydrogen Fluoride) and other compounds, you will love this comprehensive guide.
So let’s start without further introductions.
The Definition:
These are the attractive and repulsive forces that are present within the molecules of a substance. And these forces are related to most of the physical and chemical properties of matter.
Pretty simple, isn’t it? Now here comes the fun part.
Important Note: IMF’s are also referred to as ‘relatively weaker forces’ because they are comparatively weaker to the forces within molecules due to covalent bonding.
Now, you need to know about 3 major types of intermolecular forces. These are:
- London dispersion forces (Van der Waals’ forces)
- Permanent dipole-dipole forces
- Hydrogen Bonding
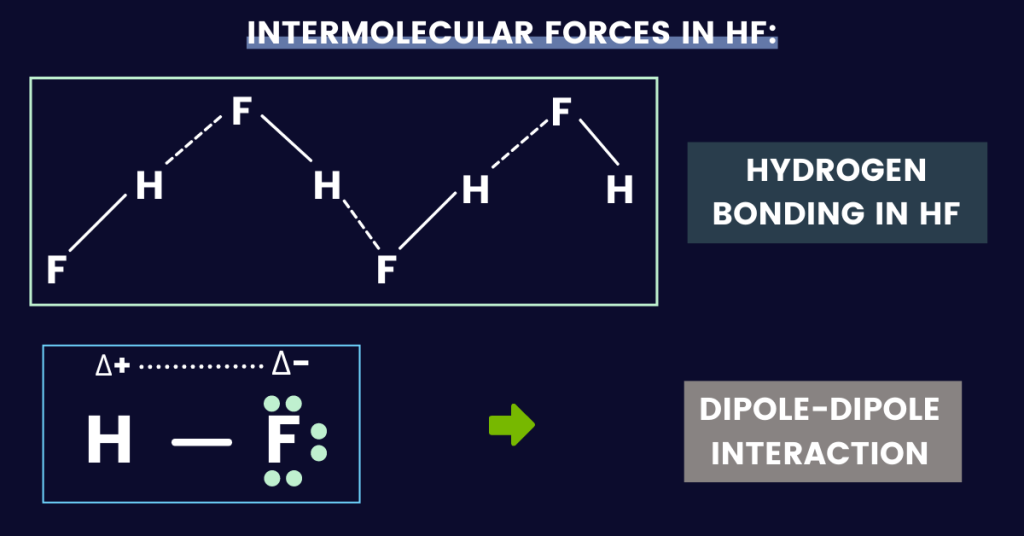
Quick answer: The major “IMF” in hydrogen fluoride (HF) is hydrogen bonding (as hydrogen is bonded to fluorine). Since the molecule is polar, dipole-dipole forces also exist along with London dispersion forces (Van der Waals’ forces).
Before taking a look at each of them in detail, here are some important concepts you need to know.
Electronegativity:
When an atom is covalently bonded to another atom, then its ability to attract an electron pair is known as electronegativity. Let me explain.
In simple words, it is a chemical property that allows an atom to attract electrons towards itself. For example, here are the electronegativity values of Oxygen and Sulfur:
Oxygen (EN) = 3.44
Sulfur (EN) = 2.58
From the information above, you can say that oxygen is more electronegative than sulfur because its “EN” value is greater.
Note that the electronegativity increases as you:
- As you move across the periodic (from group 1 to 17).
- As you move up a group.
And remember that oxygen, nitrogen and fluorine are the MOST electronegative elements. Now, let’s talk about polarity.
Polarity:
Polarity refers to the presence of an electric charge (positive and negative) around an atom or molecule. And it is important to know about it to better understand our topic.
First of all, let’s talk about non-polar molecules.
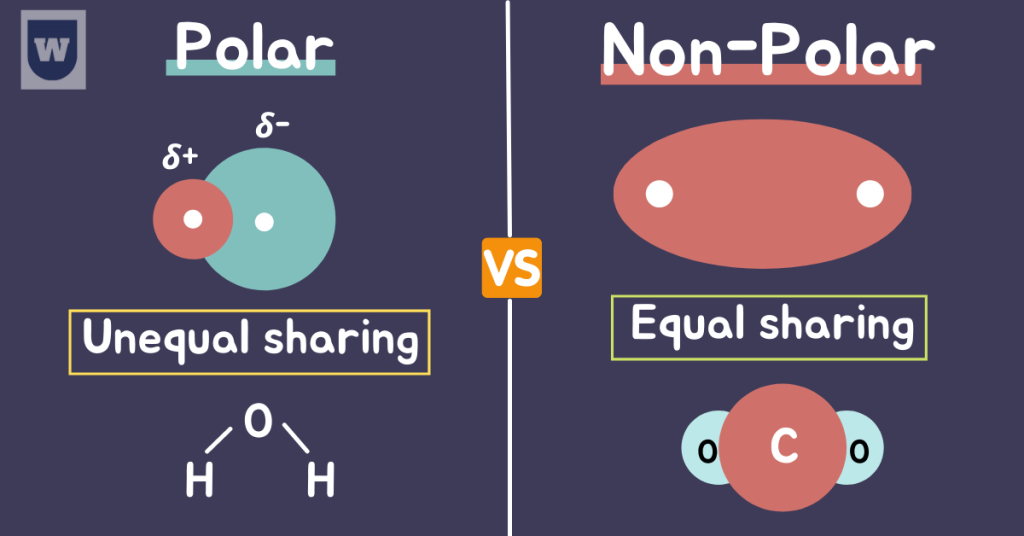
In non-polar molecules, the electrons are equally shared between the atoms of a compound. Some examples are:
- Chlorine (Cl2)
- Hydrogen (H2)
- Bromine (Br2)
Note: If the difference in electronegativity is less than 0.4, the compounds are generally considered to be non-polar.
But as the difference in electronegativity increases, the bond becomes MORE polar.
In simple words, the unequal sharing of electrons between the atoms leads to the formation of partial positive (δ+) and partial negative charges (δ-) on atoms.
This simple technique will help you better understand this concept:
- The atom that attracts electrons MORE strongly → Partial negative charge (δ-)
- The atom that attracts electrons LESS strongly → Partial positive charge (δ+)
The molecules with these charges are called polar molecules. With this, it is time to move on to our next topic.
Pretty simple, isn’t it?
Types of intermolecular forces:
Here are some types of forces you need to know about:
- Van der Waals’ forces
- Permanent dipole-dipole forces
- Hydrogen bonding
Let’s take a look at each of them in detail.
Van der Waals forces:
The BEST thing about this force is that there are multiple ways you can refer to it: Induced dipole force or London dispersion force.
These are the different types of Van der Waals’ forces.
And this force is present between ALL atoms or molecules. Here are some concepts you should learn.
In a non-polar molecule, the electron charge cloud (electrons surrounding the nucleus of an atom) is constantly moving. Due to this movement, the electron cloud gets closer to one side of the molecule than the other.
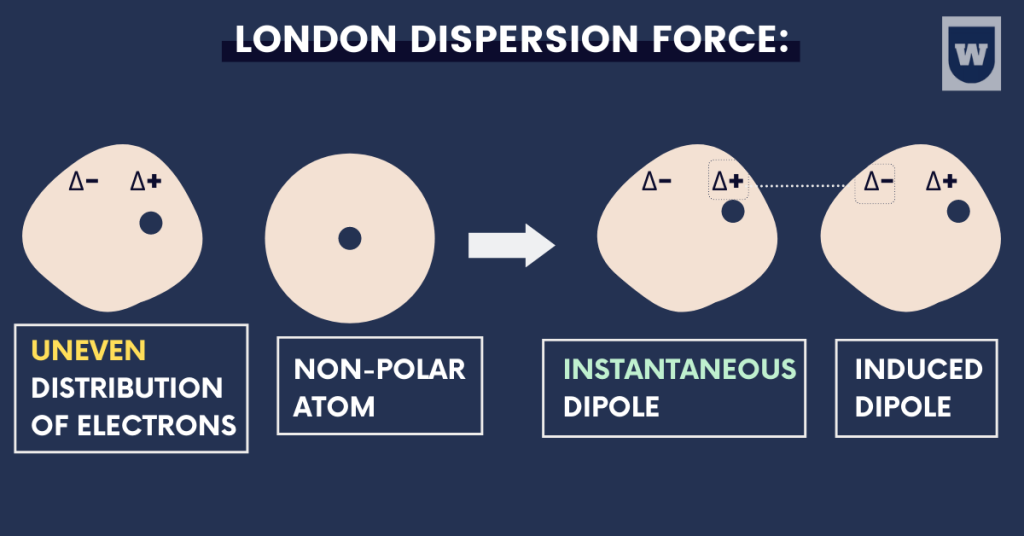
As a result, this part of the molecule becomes slightly negative for a while. And the other part becomes slightly positive. This is called a temporary dipole.
This is known as the London dispersion force of attraction.
And, do you know how this force increases? This force increases as the number of electrons and protons increase in a molecule.
Note: This is the weakest intermolecular force.
Summary:
- Electron charge cloud moves.
- Uneven distribution of the electron cloud forms dipole.
- This results in temporary dipole (induced dipole) forces.
This takes us straight to the next topic, permanent dipole-dipole forces.
Permanent dipole-dipole forces:
Quick question: What is meant by permanent dipoles?
For temporary dipole forces, we learned that they are temporary (due to the uneven distribution of the electron cloud).
But permanent dipole-dipole forces are found between polar molecules because their dipole is permanent. Let me explain.
Let’s take propanone as an example. The formula is:
(CH3)2CO.
In this compound, the carbon atom bonded to the oxygen atom has a charge of partial positive (δ+). And, oxygen has a charge of partial negative (δ-). These charges attract each other.
This is because carbon and oxygen have a permanent dipole.
Summary: Dipole-dipole force is the electrostatic force between (permanent) polar molecules. In this interaction, the positive end of the molecule is attracted to the negative end.
Some other examples are:
- Water (H2O): From the formula, you can say that two hydrogen atoms are bonded to one oxygen atom.
Now, here is something you should focus on.
The O-H bond has a permanent dipole. Hydrogen is partially positive, while oxygen is partially negative. This attraction leads to dipole-dipole interaction.
- Hydrogen chloride (HCl): In this molecule, the hydrogen atom is partial positive, while the chlorine atom is partial negative.
So when two HCl molecules are brought together, the H of one molecule attracts the Cl of the other and vice versa.
Pretty simple, isn’t it?
Now let’s talk about hydrogen bonding.
Hydrogen Bonding:
Quick question: Is hydrogen bonding the strongest intermolecular force?
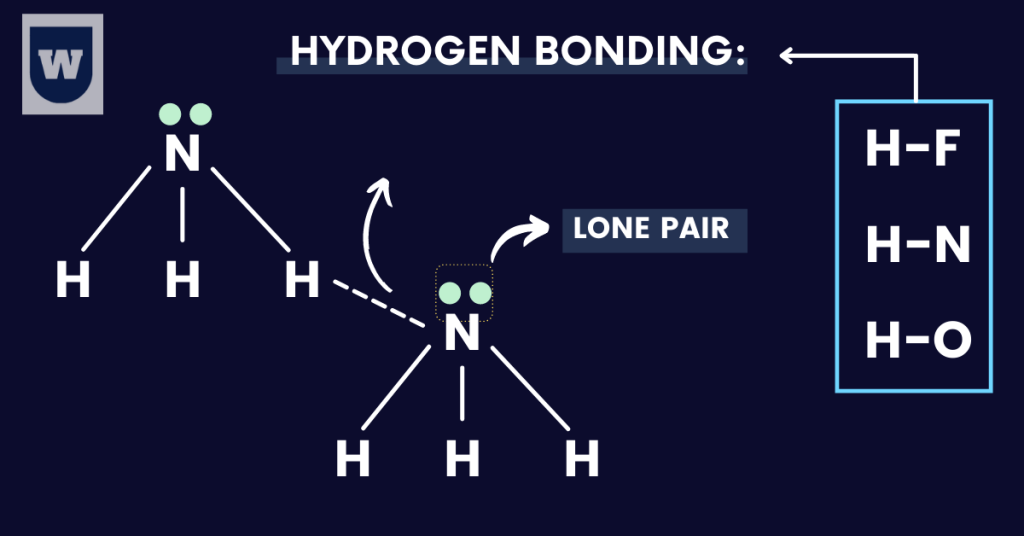
Yes, it is! Let me explain.
In this type of bonding, the hydrogen atom is bonded to a HIGHLY electronegative atom. These are:
- Oxygen (O)
- Fluorine (F)
- Nitrogen (N)
To make this concept easy for you, here are the TWO requirements for hydrogen bonding:
- The hydrogen atom should be covalently bonded to one of these atoms: N, F and O.
This is because these elements are highly electronegative, and leave the hydrogen atom with a positive dipole.
- The electronegative atom (N, F or O) in the second molecule has a lone pair of electrons (not involved in bonding).
Let me explain.
You have two water molecules, H2O and H2O. The oxygen atom of the second H2O molecule should have a lone pair for hydrogen bonds to form.
Simple?
Further reading:
Atomic structure for Oxygen (O2) | Best Guide
Ionic Bonding of NaCl (Sodium Chloride) | Made Simple
Electrolysis explained | A definitive guide
Let’s take a look at some examples to better understand this topic.
Hydrogen bonds in water (H2O):
In a water molecule, we have two hydrogen atoms and two lone pairs per molecule. Since the hydrogen atom is bonded to a highly electronegative oxygen atom, we say that water has hydrogen bonds.
Now, let’s talk about HF.
Hydrogen bonds in HF (Hydrogen Fluoride):
In an HF molecule, the hydrogen atom is bonded to the fluorine atom that has three lone pairs of electrons.
If you recall the above information, hydrogen fluoride has hydrogen bonds because hydrogen is bonded to the fluorine atom.
Note: Since Fluorine has the highest electronegativity value, it forms the STRONGEST hydrogen bond.
Intermolecular forces in HF (Hydrogen Fluoride):
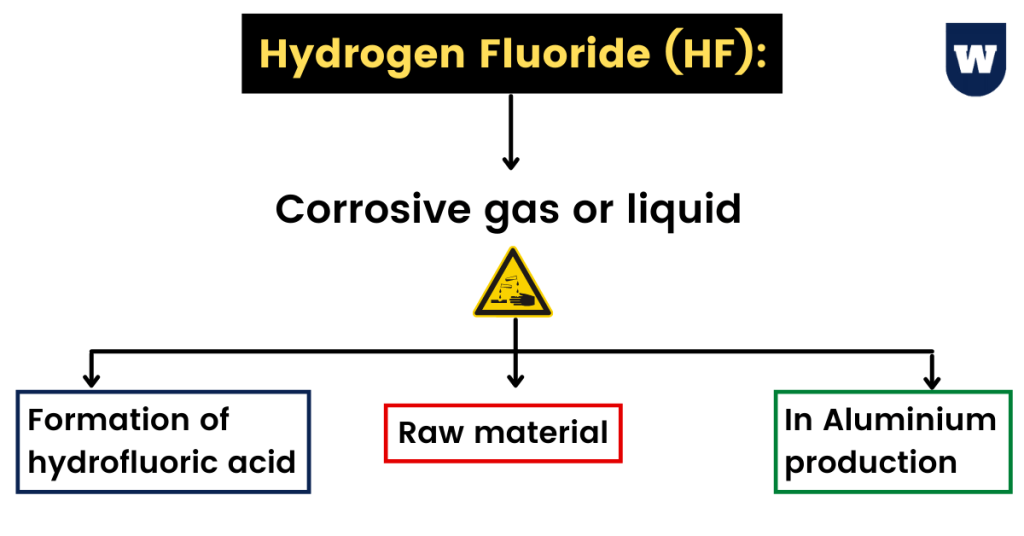
Do you know that HF is a corrosive gas or liquid made up of one hydrogen and fluorine atom? And, it is colourless as well.
Before talking about the forces, here are some concepts you need to know.
First of all, when HF is dissolved in water (H2O), hydrofluoric acid is formed. Plus, HF is a raw material used in industries for the manufacture of aluminium, gasoline and refrigerants.
With this, it is time to move on and talk about the forces (intermolecular) in hydrogen fluoride:
- Van der Waals’ forces
- Dipole-dipole forces (permanent dipole force)
- Hydrogen bonding
Van der Waals forces:
In HF, we have Van der Waals forces of attraction. These are the weak forces of attraction between electrically neutral molecules, that collide with each other.
You should also know that this force is caused due to the temporary attraction between the “electron-poor” region of one molecule, and the “electron-rich” region of the other.
Pretty simple, isn’t it?
Hydrogen bonding in HF:
In an HF molecule, hydrogen bonding occurs due to a partially positive hydrogen atom bonded to a highly electronegative fluorine atom.
Now you might be wondering, why does hydrogen have a partially positive charge?
Well, the fluorine atom pulls the electron from hydrogen towards itself. This makes hydrogen partially positive as it is giving away an electron.
Due to this, the interaction between the partially positive hydrogen atom, and the partially negative fluorine atom results in the formation of a hydrogen bond.
Now, let’s talk about dipole-dipole interactions.
Dipole-dipole interactions:
These are the simplest forces to understand. Here’s why.
This force holds the molecules together. From the information above, you know that hydrogen fluoride is a polar covalent molecule.
Plus, fluorine has a partial negative charge, while hydrogen has a partial positive charge. In simple words, electrons spend more time on F (fluorine).
So here is what you need to know.
These partial charges attract each other, and this attraction is known as dipole-dipole forces.
So these are the 3 major forces you should know about. Now, let’s talk about some other molecules for you to better understand this topic.
Intermolecular forces of NH3 (Ammonia):
Do you know that ammonia (NH3), a colourless and pungent-smelling gas, has a nitrogen atom covalently bonded to three hydrogen atoms?
Now, here are some other details you need to know.
Dipole-dipole forces:
If you look at the molecular geometry of ammonia (N3), you will notice that the nitrogen atom (bonded to 3 hydrogen atoms) have a lone pair as well.
Here is what happens.
We know that nitrogen is more electronegative than hydrogen. In simple words, we have a negative charge “around the nitrogen atom”. And a positive charge “around the hydrogen atom”.
These opposite charges make ammonia (NH3) polar.
Hydrogen Bonding:
To understand hydrogen bonding, just remember that this type of bonding ONLY occurs in the following cases:
- H-O
- H-F
- H-N
In the case of ammonia, NH3, nitrogen is bonded to hydrogen. Due to a large difference in electronegativity, we say that hydrogen bonds form.
And recall from the information above, we need to have at least one lone pair for hydrogen bonding to occur.
Note that London dispersion forces are ALWAYS present. But they vary in strength.
Intermolecular forces in HCl:
- Dipole-dipole forces:
HCl is a polar molecule. So, the chlorine atom being more electronegative holds a partial negative charge. And, the less electronegative H atom holds the partial positive charge.
This attractive force between the opposite charges is known as dipole-dipole interaction (electrostatic force). Here is a question for you.
Question: Why is the boiling point of HCl higher than F2, when both have the same number of atoms and molecular mass?
Answer: The comparatively strong dipole-dipole interactions in HCl molecules keep them “stick together”. But, the weaker dispersion forces in F2 (non-polar) are easily overcome.
Moreover, we have London dispersion forces in HCl as well.
Simple, isn’t it?
Wrapping up:
With this, our topic about the intermolecular forces in HF (hydrogen fluoride) has come to an end.
Now, let me hear from you.
Which part of this topic (intermolecular forces) do you enjoy reading the most? Or is there one you find challenging? Either way, do let me know.
And here is a quick question for you: What is the difference between intermolecular and intramolecular forces?
To conclude, we talked about hydrogen bonding, temporary dipole and permanent dipole forces. Thank you for reading and staying with me till the end.
