You will love this comprehensive guide if you want to learn about the states of matter.
These are the exact notes I used to ace my Chemistry exams. But first, I have a question for you.
Are you familiar with the most common states of matter on earth?
YES!
I am talking about solids, liquids, and gases. Bose-Einstein condensate and Plasma are some additional states of matter.
However, you are only required to know about solids, liquids, and gases.
Here’s what you need to know.
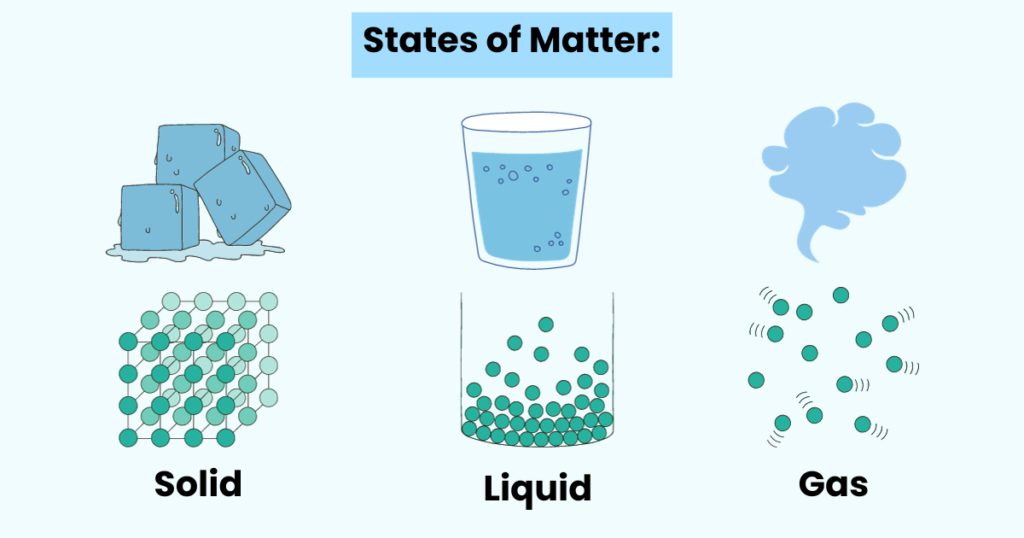
States of Matter:
Try pouring some water into a glass. What do you notice?
Water takes the shape of the glass. Can you tell why that is so?
This is because liquids do not have a fixed shape due to their “not so strong internal structure.”
To understand all this, we must know the properties of solids, liquids, and gases.
The table below summarises all this for you:
| Property | Solid | Liquid | Gas |
|---|---|---|---|
| Shape | Fixed | Not fixed | Not fixed |
| Volume | Fixed | Fixed | Not fixed |
| Molecular motion | Low | Intermediate | High |
| Compressibility | No | Low | High |
| Density | High | Intermediate | Low |
Let me explain all this to you in detail.
Solids:
The particles (atoms, ions, or molecules) are tightly packed in solids, so they do not move much.
This arrangement of particles is the reason behind the strong forces between the particles.
That’s why we say that solids have definite volume and shape.
Here’s a quick summary for you:
- Definite shape
- Definite volume
- High density
- Lowest kinetic energy (as they are tightly packed and only vibrate in place)
This takes us straight to liquids.
Liquids:
As compared to solids, the particles in liquids are more loosely packed.
This allows the particles to flow around each other, giving liquids an indefinite shape.
Now you know why a liquid takes the shape of a container.
You should also know that liquids are nearly incompressible fluids because of the strong force of attraction between the particles.
So, here’s a quick summary for you:
- Particles slide past one another (no definite shape)
- Not easily compressible
- Moderate level of kinetic energy, space, and intermolecular forces
Now, let me introduce gases to you.
Gases:
You know that liquids are incompressible fluids. What about gases?
Yes! They are not.
A gas is a compressible fluid. Plus, the particles in a gas have a great deal of space between them.
That’s why they have a high kinetic energy.
Interesting!
A gas has no definite shape or volume and takes the shape of a container.
Here’s the takeaway from all of the above discussion.
Particles in a:
- Gas: Well separated (no proper arrangement)
- Liquid: Close together (no regular arrangement)
- Solids: Regular pattern (tightly packed)
And particles in a:
- Gas: Move freely at high speeds
- Liquids: Vibrate, move, and slide past each other
- Solids: Vibrate but do not move from place to place
This takes us straight to the next topic.
How Do States of Matter Change?
Take a look at the image below:
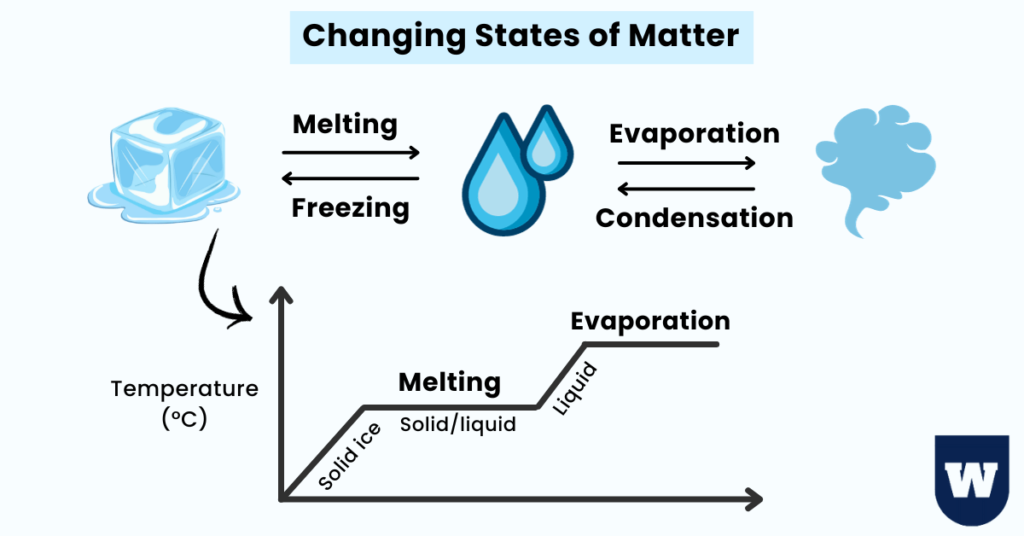
Have you ever seen ice cubes melt into water or a liquid boiling into vapours?
This means that you already know about the changes in states of matter.
Before discussing this, I want to introduce the kinetic particle theory.
According to this theory, changes in the state of matter occur when energy is added or removed from a substance.
For example, adding energy to a solid allows the particles to move more, pushing them apart.
As a result, bonds weaken, and the solid can change into liquid or gas.
Simple, isn’t it?
So, here’s what you need to know.
Change of Phase Between Solids and Liquids:
You want to form ice cubes. You take a tray, fill it with water, and place it into a refrigerator’s freezer compartment.
What’s next?
Freezing:
Heat flows from the warmer tray to the colder air (freezer). This heat transfer continues until the tray loses all its energy.
You should know that heat naturally flows in one direction – hot to cold.
In this way, water (liquid) is converted to ice (solid).
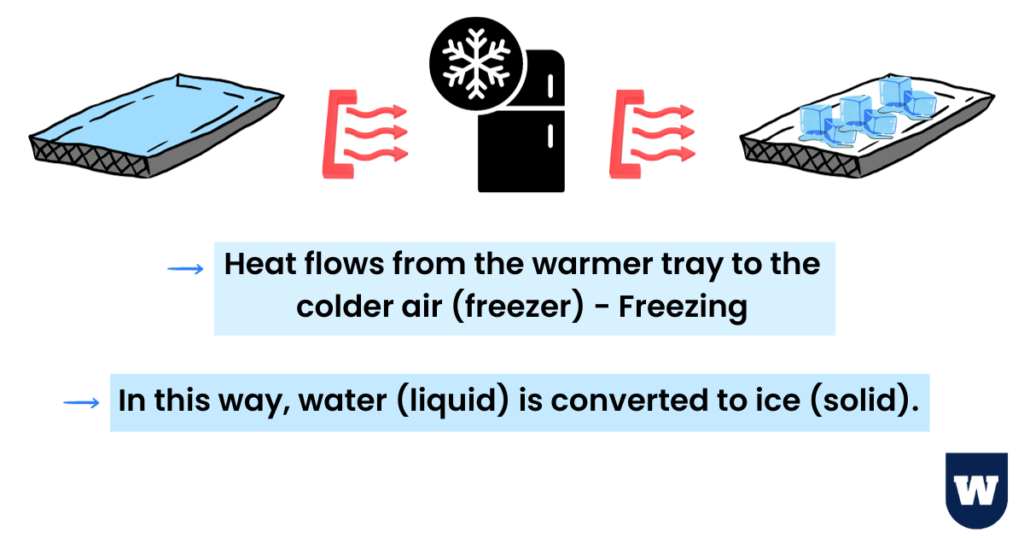
This is what we call freezing.
The temperature at which freezing occurs is known as the freezing point.
Do you know the freezing point of water? Comment down the correct answer.
Melting:
When you take out the ice cubes from the refrigerator, what happens?
Heat flows from the surrounding (warmer air) to the ice cubes. This energy absorbed by the ice cubes weakens the force of attraction.
Thanks to all this, the particles are free from the “ice-like grip”.
This is melting – changing from a solid to a liquid state.
Recall that this occurs when heat is added to the system.
Note: The conversion of a solid straight to a gas is known as sublimation.
This takes us to the next point.
Change of Phase Between Gases and Liquids:
Here, we are also going to discuss the addition or removal of heat.
Imagine you fill a container with cold tap water and then heat it on a hot cooktop. What will happen?
You are right.
Heat flows from the cooktop to the pot, which water then absorbs.
Here’s what happens next:
Boiling:
When liquid particles gain energy, they start to move faster. This is because the thermal (heat) energy is converted into kinetic energy.
Over time, the particles will have enough energy to overcome the forces of attraction between them and convert into the gaseous phase.
You should know the temperature at which a liquid boils is known as the boiling point.
Important Note:
Do you know the difference between boiling and evaporation?
| Boiling | Evaporation |
|---|---|
| Unnatural process (the liquid gets heated up) | Normal process |
| Quicker | Slower |
| Can occur throughout the liquid | Occurs only from the surface of the liquid |
| Does not lead to cooling | Leads to cooling |
Remember that the conversion of liquid to gas is vaporisation (evaporation or boiling).
Now, let’s jump to the next point.
Condensation:
The reverse of vaporisation!
Have you ever noticed the fog in the mirror after a hot shower? This is because of condensation.
Let me explain.
When gas loses energy, the particles come together to form a liquid. In the same way, steam from hot water can condense on a colder surface (mirror).
In short, the conversion of gas into liquid is known as condensation.
With this, it is time to move on to the next topic.
EXAM POINT: When asked to describe or explain the state changes, linking your answer to the kinetic particle theory is essential.
This takes us to the heating and cooling curves.
Heating Curve:
How would the temperature of a block of ice change when a Bunsen burner is placed underneath it?
For that, let’s take a look at its heating curve:
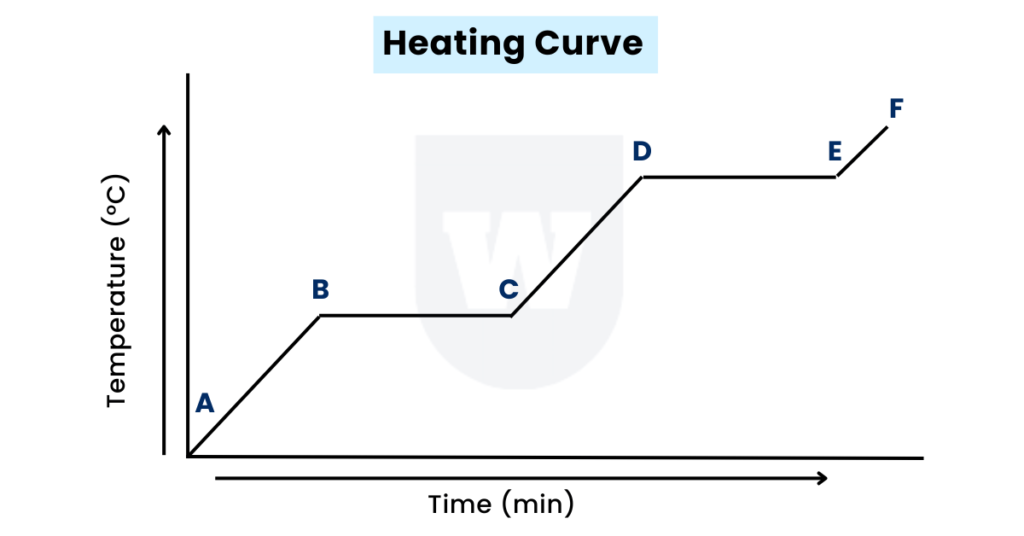
As the name suggests, a heating curve is a graph of temperature against time.
To better understand this, let’s break this down.
Phases in a Heating Curve:
- A-B: The block of ice has started to gain some heat. Therefore, the temperature is rising.
Recall that heat flows from hotter (bunsen burner) to colder objects (ice).
- B-C: Whenever you see a horizontal flat part, remember that it represents a state change.
This change converts the ice (solid) to water (liquid) – melting.
These plateaus are also known as phase changes.
You might be wondering why the temperature is constant during a state change.
Here’s why.
The temperature of any substance is related to its average kinetic energy. As the temperature increases, the kinetic energy also increases.
But the particles have “potential energy” when discussing a phase change. So, the heat during this stage is not converted into kinetic energy.
Instead, it is converted to potential energy.
So, instead of the temperature rising, a state change occurs.
That’s why both solid and liquid states exist at this point.
But at the end of this stage, the solid is completely converted into a liquid.
- C-D: Now, the temperature of the liquid water starts to rise again as heat is converted to kinetic energy.
- D-E: It’s time for a state change. YES! That’s what a horizontal line represents.
This is boiling – liquid to gas.
The temperature stays the same while a substance boils as the energy gained is used to break the attractive forces between the particles.
Now, let’s talk about a cooling curve.
Cooling Curve:
You’ve guessed this one right!
A cooling curve shows the temperature of a substance as it is cooled (opposite to heating curves).
Like heating curves, a flat line represents a state change when the temperature remains constant.
Note that melting and freezing occur at the same temperature. Energy is removed during freezing, and energy is absorbed during melting.
Pretty simple.
Effect of Temperature on Volume of Gas (Charle’s Law):
Have you ever seen a flying hot air balloon?
Let’s understand how this happens.
According to Charles’s law, gases will expand when heated.
This means that temperature and volume (of container) are directly proportional.
If I talk about the kinetic particle theory, the increase in temperature increases the kinetic energy of particles.
As a result, the particles collide more frequently with the walls of the container. This results in an increase in volume.
Returning to the hot air balloon, a torch heats the air inside the balloon (making the air less dense than the surrounding air).
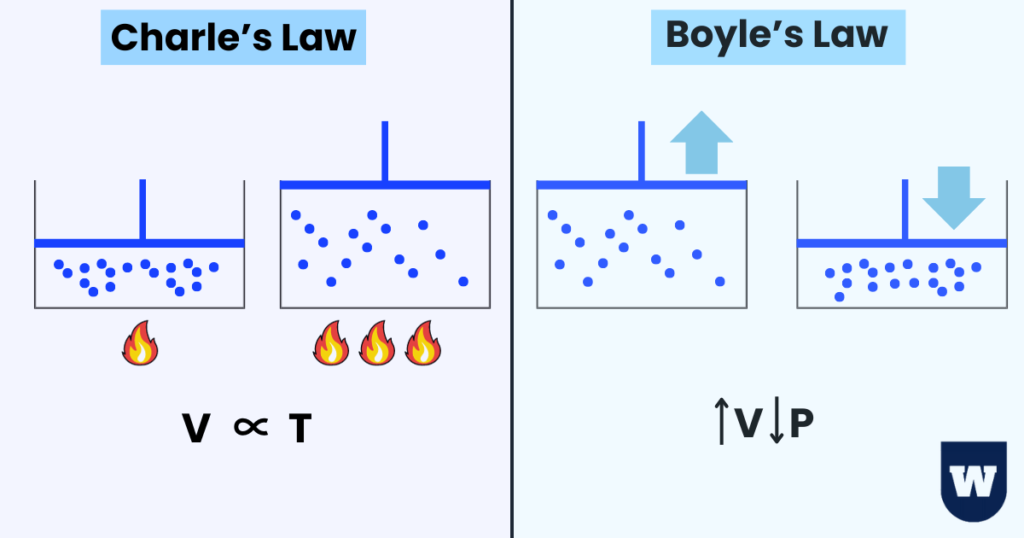
In short:
V ∝ T (when pressure is kept constant)
This takes us to Boyle’s law.
Effect of Pressure on Volume of Gas (Boyle’s Law):
The Boyle’s law states that:
“At a constant temperature, the volume of a fixed mass of gas is inversely proportional to its pressure.”
Let’s understand this.
Fun fact: The human breathing mechanism exemplifies Boyle’s law.
If you increase the volume of a container, the pressure will decrease because there will be fewer collisions of the particles with the walls of the container.
Recall that the force the particles apply on the walls of a container is called pressure.
So:
V ∝ 1/P (when temperature is constant)
This relation gives us the formula:
P1V1 = P2V2
This takes us straight to the next topic.
Diffusion:
If you spray a perfume in the air, the particles of the perfume spread through the air.
That’s why the fragrance will spread even if you spray at the corner of a room. This is what we call diffusion.
Diffusion is the process resulting from the random motion of molecules in which there is a net flow of matter from a region of high concentration to a region of low concentration.
Now, let’s talk about the factors affecting diffusion.
Factors Affecting Rate of Diffusion:
- Molecular mass (for gases):
In simple words, the molecular mass is the mass of the particles of a gas.
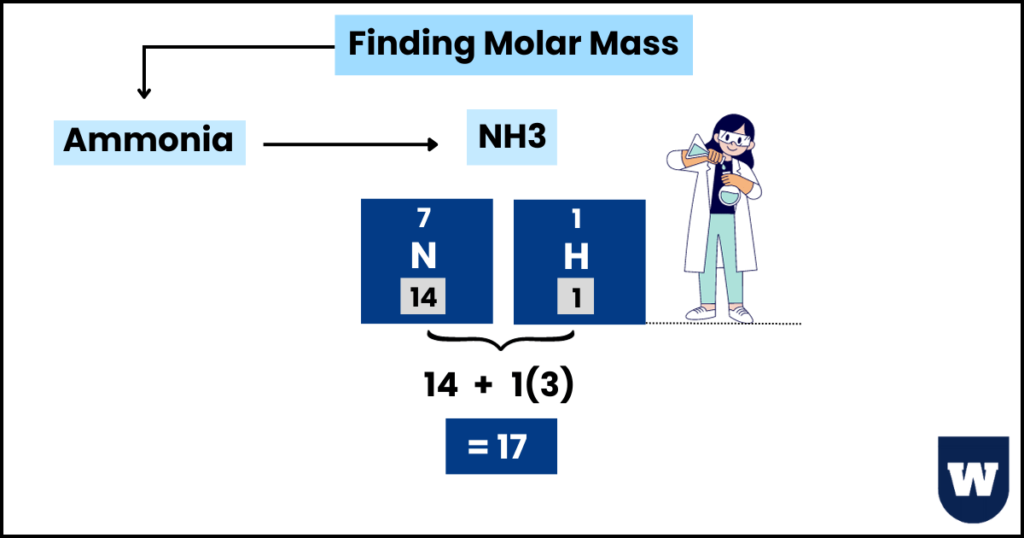
Let’s understand this with the help of an example:
- Methane (CH4):
The molecular mass of methane is → (12) + (1×4) = 16
- Ammonia (NH3):
The molecular mass of ammonia is → (14) + (1×3) = 17
Note that the rate of diffusion of methane is greater than ammonia. So, here’s the takeaway.
Gases with lower molecular masses diffuse faster than those with higher molecular mass.
This takes us to the next point.
- Temperature:
Do you know why increasing the temperature increases the rate of diffusion?
Let’s understand this through the kinetic particle theory.
As the temperature increases, the average kinetic energy of the molecules in a fluid also increases.
As a result, they start to move faster by gaining more energy. So, increasing temperature increases the rate of diffusion.
Simple, isn’t it?
Wrapping Up:
Now, I turn it over to you.
If you have any questions regarding the “states of matter,” do let me know.
Here are some other CIE O Level Chemistry chapter notes:
Unit 2: Atoms, elements and compounds
Unit 6: Chemical Reactions
Unit 7: Acids, bases, and salts
Unit 9: Metals
Unit 10: Chemistry of the Environment
Unit 12: Experimental Techniques and Chemical Analysis
Happy learning!
