Around 85% of students find stoichiometry and moles challenging.
If you are one of them, you will love this comprehensive guide.
Plus, this is the exact resource I used to crack my CIE.
Here’s all you need to know.
Molecular Formula:
I like to call it the “regular formula.”
Have you ever heard of water?
Of course!
It’s H₂O. That’s the molecular formula of water.
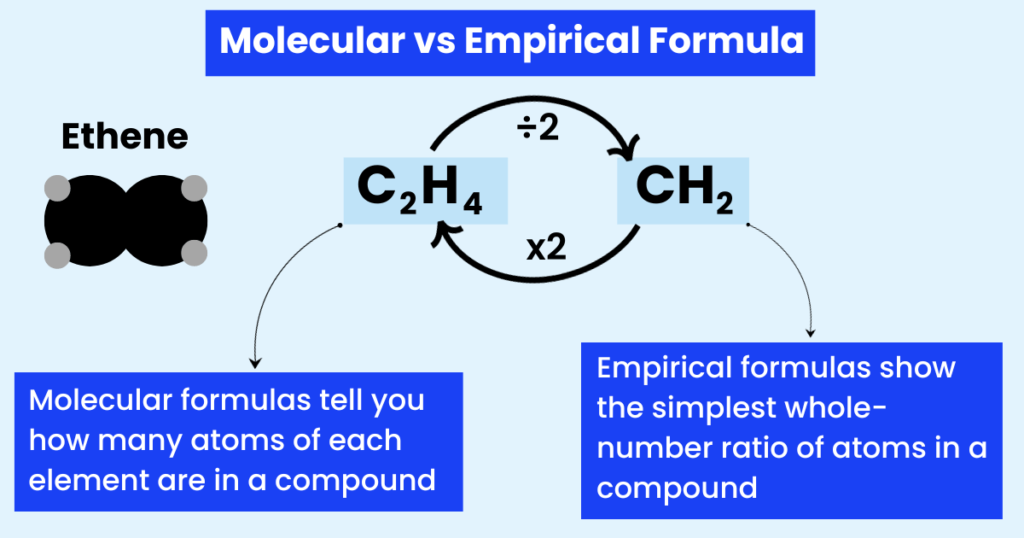
In simple words:
The molecular formula tells you how many atoms of each element are in a compound.
Coming back to the water, H₂O shows that water has:
- 2 hydrogen atoms
- 1 oxygen atom
Pretty useful!
Here are some other examples:
| Compound: | Molecular Formula: |
|---|---|
| Methane | CH4 |
| Ethane | C2H6 |
| Hydrogen Peroxide | H2O2 |
What’s the takeaway?
The molecular formula of a compound tells the precise number of each atom.
Here’s how Cambridge mentions this in the syllabus outcome:
“The number and type of different atoms in one molecule.”
This takes us straight to the next topic.
Empirical Formula:
You should remember the empirical formula as the “simplest formula.”
Here’s why.
Let’s say you have a ratio:
4:8
Can you simplify it?
Of course!
1:2 (that’s the answer that you would give me)
That’s exactly an empirical formula.
It is the smallest whole-number ratio of different atoms or ions in a compound.
Here’s an example.
- Glucose (C6H12O6)
If you notice, carbon, hydrogen, and oxygen are in a ratio:
6:12:6
If we divide them by 6, we will get the following:
1:2:1 (the simplest whole number ratio).
Therefore, the empirical formula of glucose will be CH2O.
Let me share some other examples:
| Compound: | Molecular Formula: | Empirical Formula: |
|---|---|---|
| Ethane | C2H6 | CH3 |
| Hydrogen Peroxide | H2O2 | HO |
| Cyanuric triazide | C3N12 | CN4 |
Now, I have a question for you.
What is the empirical formula of water (H2O)?
YES!
It’s H2O.
Sometimes, the empirical formula is the same as the molecular formula.
This takes us to the next topic.
How To Calculate Empirical Formula?
To calculate the empirical formula, we are going to do three simple steps:
- Write the given percentage of each element.
- Use the periodic table and divide the percentage of each element by its mass number.
- Divide each by the smallest number and round to the nearest whole number.
Here’s a past paper question.
Past Paper Question (Easy):

Let’s solve this.
- Step 1: Write the given percentage of each element
In this case, we have three elements:
- Carbon
- Hydrogen
- Oxygen
So, according to the data given in the question, it will be:
C → 54.54
H → 9.09
O → 36.37
This takes us to the second step.
- Step 2: Use the periodic table and divide the percentage of each element by its mass number
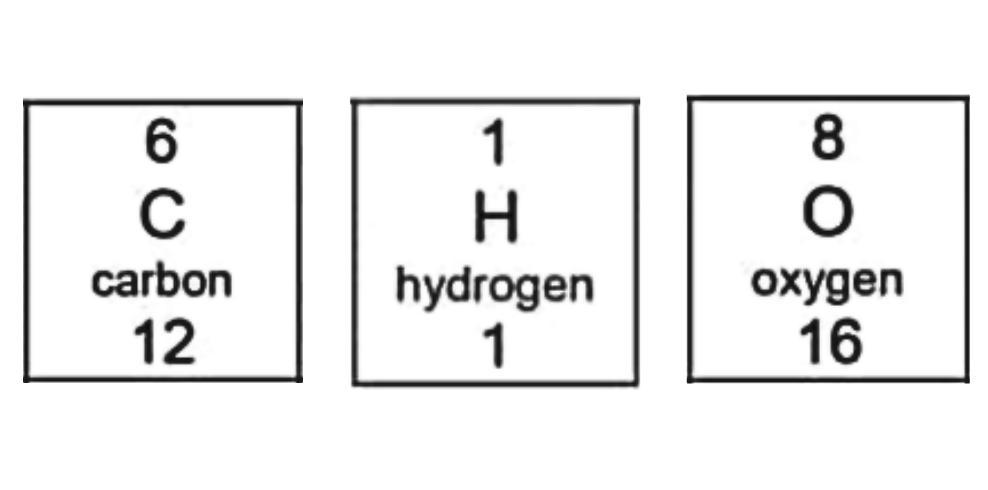
As you can see, the mass number (atomic mass) of each element is:
- Carbon: 12
- Hydrogen: 1
- Oxygen: 16
Therefore:
C → 54.54/12 = 4.54
H → 9.09/1 = 9.09
O → 36.37/16 = 2.27
This takes us to the final step.
- Step 3: Divide each by the smallest number and round to the nearest whole number
As you can see above, 2.27 is the smallest among the three.
Therefore, we will divide each by 2.27.
C → 4.54/2.27 = 2
H → 9.09/2.27 = 4
O → 2.27/2.27 = 1
So, the empirical formula is:
C2H4O
Simple!
Past Paper Question (Hard):
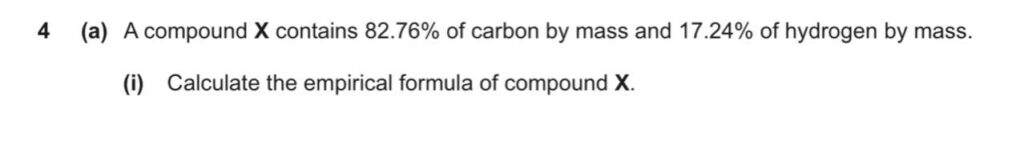
Here’s the solution.
- Step 1: Identify each element and note down the data given.
In this question, we are only dealing with Carbon and Hydrogen.
So:
Carbon → 82.76
Hydrogen → 17.24
This takes us to the second step.
- Step 2: Divide each by its mass number.
Carbon → 82.76/12 = 6.90
Hydrogen → 17.24/1 = 17.24
With this, let’s move on to the final step.
- Step 3: Divide each by the smallest number and round to the nearest whole number.
Carbon → 6.90/6.90 = 1
Hydrogen → 17.24/6.90 = 2.5
The Problem (And the Fun Part):
Look, we need whole numbers.
You cannot simply round off 2.5 to 3 – only a slight margin is allowed.
For instance, 2.8 or 2.9 can be rounded to 3 (BUT NOT 2.5).
In this case, we are simply going to:
Multiply the mole ratios of each element by 2 to obtain a whole number.
Therefore:
Carbon : Hydrogen
(1 : 2.5) x 2
In this way, you get:
C:H = 2:5
So, the empirical formula is:
C2H5.
This is how you can tackle problems like this.
Finding Formula of Ionic Compounds:
In the previous chapter, I explained how ionic compounds are formed.
To recap, an ionic compound is formed through electron transfer. So, we have to deal with cations and anions.
To find the formula of an ionic compound, we are going to do three simple steps:
- Identify the cation and its charge (metals – left side of the periodic table).
- Identify the anion and its charge (non-metals and halogens).
- Cross-multiply their charges.
Let’s understand this with the help of an example.
Finding the Formula of Calcium Chloride:
- Step 1: Identify the cation
You should know that cation is the most electropositive (least electronegative ion).
So, a cation is positively charged.
In simple words, it is the one that loses electron(s).
As I mentioned above, metals lose electrons easily. So, in this case, calcium is the cation.
The charge on calcium is +2. In short, the cation is Ca+2
Exam Tip: The charge on a cation is the same as its group number in the periodic table.
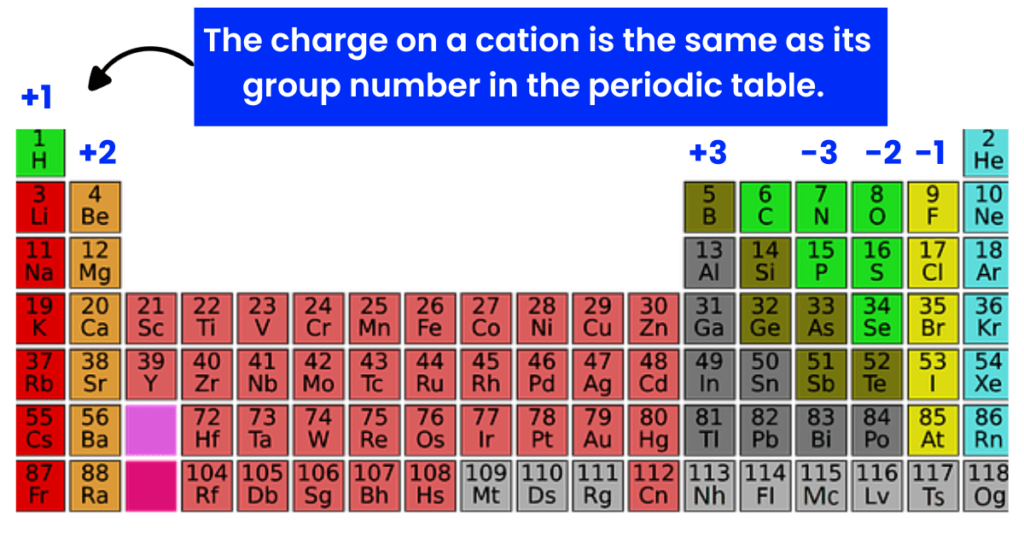
This takes us to the next step.
- Step 2: Identify the anion
As you already know, an anion is a negatively charged ion.
In this case, chlorine is the anion as it gains an electron to become stable.
So, the anion is Cl–.
This takes us to the last step.
- Step 3: Cross-multiply / exchange the charges
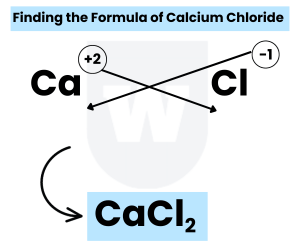
Here’s what we have:
Ca+2 and Cl–
After cross-multiplying the charges, we get the formula of calcium chloride:
CaCl2
This table below will help you remember some important cations and anions (with their charges):
| Cations: | Anions: |
|---|---|
| Iron (||): Fe+2 | Hydroxide: OH- |
| Calcium: Ca+2 | Chloride: Cl- |
| Magnesium: Mg+2 | Sulfate: SO42- |
| Sodium: Na+ | Carbonate: CO32- |
| Ammonium: NH4+ | Nitrate: NO3– |
| Aluminium: Al+3 | Phosphate: PO43- |
For your better understanding, the table below shows some popular ionic compounds and their formulas:
| Ionic Compound | Formula |
|---|---|
| Lithium fluoride | LiF |
| Iron (II) oxide | FeO |
| Aluminium sulfide | Al2S3 |
This takes us to the next topic.
Writing Chemical Equations:
Chemistry is all about chemical reactions.
If I ask you, what is the most efficient way of describing chemical reactions?
Chemical equations!
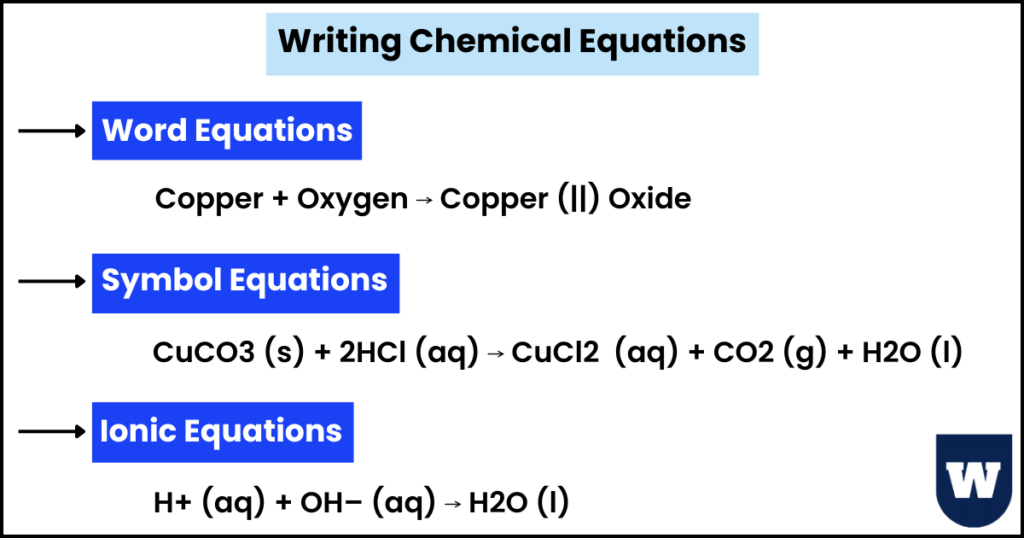
We will study three types of chemical equations:
- Word equations
- Symbol equations
- Ionic equations
Let’s take a look at each of them.
Word Equations:
Take a look at this word equation:
Copper + Oxygen → Copper (||) Oxide
(copper reacts with oxygen to produce copper (||) oxide)
What does this equation show?
As you can see, a word equation uses the names of the substances involved in a chemical reaction instead of chemical symbols.
So, we use words rather than chemical formulas.
To sum it up, word equations are:
Reactants → Products
Now, let’s talk about symbol equations.
Symbol Equations:
We use a substance’s chemical symbol or formula in symbol equations.
For example:
- Hydrogen: H2
- Oxygen: O2
- Magnesium: Mg
Exam Note: Hydrogen is H2 instead of H because atomic hydrogen (H) is highly unstable and does not exist under normal conditions.
The second important thing is using state symbols to show whether each substance is a solid, liquid, gas, or solution.
Let me explain.
What does each symbol show?
- (s): The substance is in a solid state.
- (l): The substance is in a liquid state.
- (g): The substance is in a gaseous state.
- (aq): The substance is in an aqueous solution.
Let’s take a look at an example.
Question: Use symbol and word equations to show the reaction between copper carbonate and hydrochloric acid.
Symbol Equation:
CuCO3 (s) + 2HCl (aq) ⟶ CuCl2 (aq) + CO2 (g) + H2O (l)
Compare this to the word equation:
Copper Carbonate + Hydrochloric acid ⟶ Copper Chloride + Carbon Dioxide + Water
Before moving on, here are some important points about writing equations:
- When balancing an equation, the number of atoms of each element must be the same on either side.
- Use the hit-and-trial method to balance chemical equations.
- You should write these non-metals as diatomic molecules: H2, N2, O2, F2, Cl2, Br2, I2
This takes us to ionic equations:
Ionic Equations:
In O and A-level Chemistry, you will often see this neutralisation equation:
H+ (aq) + OH– (aq) ⟶ H2O (l)
This is an example of an ionic equation.
So, ionic equations tell us about the dissolved ions that undergo a chemical reaction.
In your exam, you can be asked to write an ionic equation. So, let’s practice that.
Writing Ionic Equations:
We will write ionic equations in four simple steps:
- Write the chemical equation.
- Separate the soluble ionic compounds into their dissolved ions.
- Cancel out the common ions (spectator ions).
- Write down the net equation.
Here’s an example to better understand this.
Example 1 (Easy):
HNO3 (aq) + NaOH (aq) → NaNO3 (aq) + H2O (l)
- Step 1: Write the chemical equation.
As you can see in the question, the chemical reaction is:
HNO3 (aq) + NaOH (aq) ⟶ NaNO3 (aq) + H2O (l)
This takes us to the second step.
- Step 2: Separate the soluble ionic compounds into their dissolved ions.
Let me explain.
Let’s take NaOH as an example.
NaOH (aq) means that it has been dissolved in water. In water, the sodium and chloride ions separate.
We call this dissociation.
Dissociation is the separation of ions when an ionic compound (soluble) dissolves in water.
So NaOH (aq) means that we get:
- Sodium ion (Na+)
- Hydroxide ion (OH-)
In this way, dissociation occurs, and we get ions.
Exam Note: Dissociation only occurs for aqueous solution. So if (s) or (l) is mentioned in the question, this process will not happen.
Now, back to the question.
HNO3 (aq) is made up of two ions:
- H+
- NO3–
So, we will get:
H+ (aq) + NO3– (aq) + Na+ (aq) + OH- (aq) ⟶ Na+ (aq) + NO3– (aq) + H2O (l)
This takes us to the third step.
- Step 3: Cancel out the common ions (spectator ions)
As you can see in the above equation, we have Na+ on both sides.
Similarly, NO3- ion is also present on both sides.
We call them spectator ions because they are not involved in the reaction.
So, cut them off!
H+ (aq) + NO3– (aq) + Na+ (aq) + OH- (aq) ⟶ Na+ (aq) + NO3– (aq) + H2O (l)
This takes us to the last step.
- Step 4: Write down the net equation.
H+ (aq) + OH– (aq) ⟶ H2O (l)
This is how we write ionic equations.
Here’s one more example.
Example 2 (Tricky):
Cu (s) + 4HNO3 (aq) ⟶ Cu(NO3)2 (aq) + 2NO2 (g) + 2H2O (l)
Try this one out.
Here’s the solution.
(As the equation is already given in the question, let’s jump straight to the second step).
- Step 2: Separate the soluble ionic compounds into their dissolved ions.
Cu (s) + 4H+ (aq) + 4NO3- (aq) ⟶ Cu+2 (aq) + 2NO3- (aq) + 2NO2 (g) + 2H2O (l)
This takes us to the third step.
- Step 3: Cancel out the common ions.
Cu (s) + 4H+ (aq) + 4NO3- (aq) ⟶ Cu+2 (aq) + 2NO3- (aq) + 2NO2 (g) + 2H2O (l)
Exam Point: As you can see, we have four NO3- ions on the left side and two NO3- ions on the right side.
Therefore, we will have two net NO3- ions on the left.
So:
- Step 4: Write the net equation.
Cu (s) + 4H+ (aq) + 2NO3– (aq) ⟶ Cu+2 (aq) + 2NO2 (g) + 2H2O (l)
That’s the answer!
This takes us to the next topic.
Atomic Mass (Ar) vs Molecular Mass (Mr):
Comparing things is one of the best ways to understand something.
For example, one cup of coffee is the same as nearly ten teaspoons of sugar.
In the world of chemistry, we compare masses. This is because the mass of an atom is very small.
Like we compared coffee with sugar, scientists compare the mass of elements with Carbon 12.
Take a look at Magnesium in the periodic table.
The value “24” shows magnesium’s atomic mass (Ar) compared to 1/12th of a Carbon-12 atom.
I have a small task for you.
Look at the periodic table for the Ar of hydrogen and carbon.
1 and 12 is the answer!
Well done.
So:
Ar is the average mass of the isotopes of an element compared to 1/12th of the mass of an atom of Carbon 12.
That’s what Cambridge says.
Now you might be wondering, what does “isotopes” mean over here?
Take a look at chlorine.
It’s Ar is 35.5 (not a whole number)
Why is that so?
In nature, chlorine exists in two forms:
- Chlorine-35 (75%)
- Chlorine-37 (25%)
So, 35.5 shows the average.
Regarding other elements, such as carbon, their atomic masses are also not whole numbers. Instead, they are rounded off to make things simpler for you.
That’s good news.
Now, let’s talk about Mr.
Relative Molecular Mass (Mr):
We know that a molecule is a combination of atoms.
For example, we have water (H2O), nitrogen (N2), and calcium oxide (CaO).
In the same way, the relative molecular mass is the combination (sum) of the relative atomic mass of each element.
Here’s an example.
What is the Mr of HCl?
If you look at the periodic table, the Ar is:
- Hydrogen: 1
- Chlorine: 35.5
So the Mr of HCl is 35.5 + 1 = 36.5
Here’s another example.
What is the Mr of glucose?
We know that glucose is C6H12O6.
The relative atomic mass of each element is:
- Carbon: 12
- Hydrogen: 1
- Oxygen: 16
Now, we have six carbon, 12 hydrogen, and six oxygen atoms.
So Mr is:
(12×6) + (1×12) + (16×6) = 180
Exam Tip: The Mr is numerically equal to the mass of 1 mol of molecules in grams.
For example, the Mr of water is 18. The molar mass of water is 18g/mol.
Pretty simple!
This takes us to the next topic.
Moles (Stoichiometry):
Let’s say you have 12 eggs.
I can also say that you have one dozen eggs.
That’s the point – We use certain units to express something.
In the same way, a “mole” is a unit to measure the amount of substance.
Just like a dozen means 12, a mole means:
6.02×1023 particles (atoms, ions, or molecules).
We also call this number (6.02×1023) the Avogadro constant.
Simple.
For example:
- 1 mole of Na = 6.02×1023 Na atoms = 23g
- 1 mole of Mg = 6.02×1023 Mg atoms = 24g
- 1 mole of NaCl = 6.02×1023 NaCl molecules = 58.5g
This was all about theory.
The next thing is the calculations related to moles.
Calculations and Formulas (Moles):
Here are all the important formulas for moles:
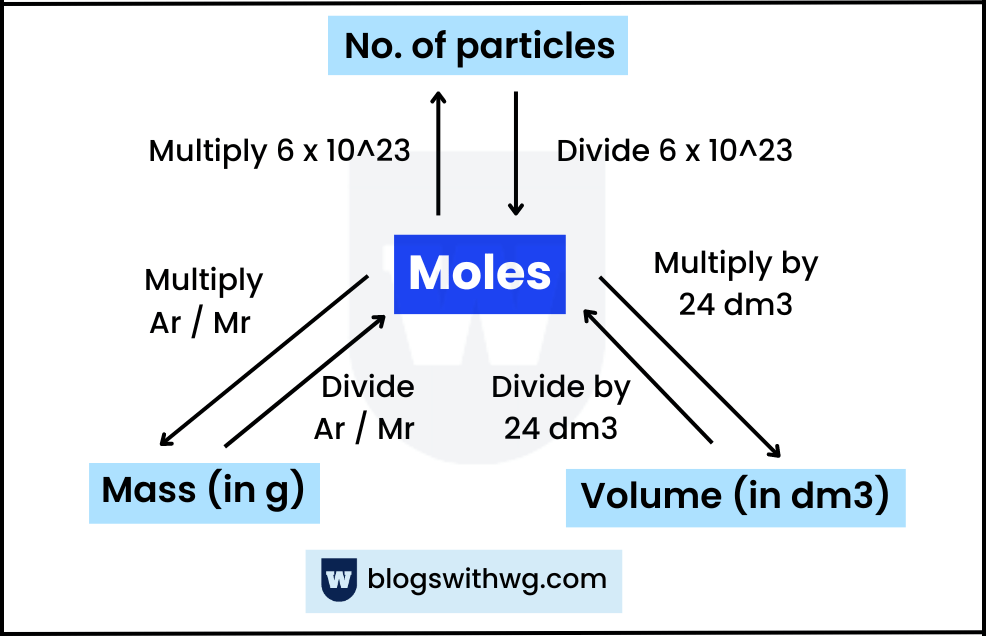
Formula 1 (Past Paper Question):
Moles = Mass (in g) / Molar Mass (Ar or Mr)
Here’s a past paper question to understand the use of this formula:
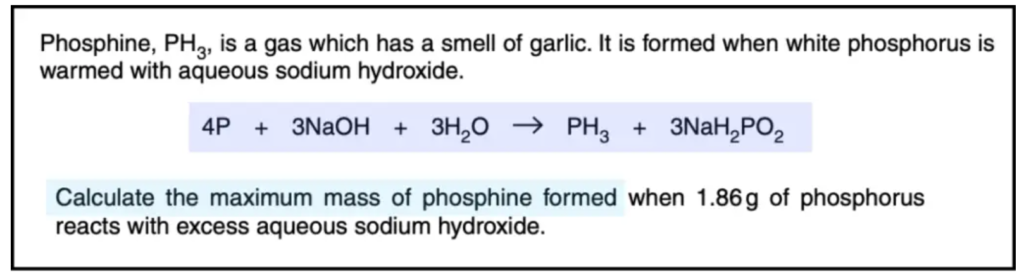
Exam Point: A balanced equation tells you the mole ratio. For example:
A + 2B → C
Let’s say that the number of moles of C is 1. What will be the number of moles of B?
2
This is because the equation shows 2:1 (B:C). In this way, we can use ratios to find out moles.
Let’s check this out.
Solution:
Give data:
- Mass of phosphorus = 1.86g
- Mass of phosphine =?
Step 1: First, let’s find out the moles of phosphorus.
Moles of phosphorus = Mass / Ar
= 1.86 / 31
= 0.06 moles of phosphorus
- Step 2: What are the moles of phosphine?
According to the balanced equation given in the question:
Phosphine : Phosphorus
= 1 : 4
Since moles of phosphine (PH3) are four times less than phosphorus:
Moles of phosphine = 0.06 / 4
= 0.015 moles (PH3)
This takes us to the final step.
- Step 3: To find the mass of phosphine:
Mass = moles x Mr
Note: Mr of phosphine (PH3) = 31 + 3 = 34
= 0.015 x 34
= 0.51 g (ANSWER)
Formula 2:
For gases, you can also find out the number of moles using:
Moles = Volume (in dm3) / 24
OR
Moles = Volume (in cm3) / 24000
Formula 3:
Moles = Concentration x Volume
Percentage Yield:
We know that the actual yield (output) is always less than the expected (theoretical) yield.
Why?
Incomplete reaction or impurities (there are other reasons as well).
So, we can use a simple formula to find out the percentage yield:
Percentage yield = (Actual yield ÷ Theoretical yield) x 100%
Here’s an example:
The decomposition of sodium carbonate forms 15 grams of sodium oxide in an experiment. The theoretical yield is known to be 19 grams. What is the percent yield of sodium oxide?
Given data:
- Actual yield = 15g
- Theoretical yield = 19g
So, the percentage yield will be:
= (15/19) x 100%
= 79%
This means that 79% of reactant is converted to product.
Exam Point: The actual yield will always be given in the question as it is found out experimentally. However, you may have to use moles to determine the theoretical yield.
This takes us to the next point.
Percentage Purity:
We can easily find out how pure a substance is.
Percentage purity = (Mass of pure substance ÷ Mass of impure sample) × 100%
Simple, isn’t it?
Wrapping Up:
Now, I turn it over to you.
Let me know if you have any questions regarding stoichiometry and moles.
Here are some other CIE O Level Chemistry chapter notes:
Unit 2: Atoms, Elements and Compounds
Unit 6: Chemical Reactions
Unit 7: Acids, bases, and salts
Unit 9: Metals
Unit 10: Chemistry of the Environment
Unit 12: Experimental Techniques and Chemical Analysis
Happy learning!
