You will love this comprehensive guide if you want to learn about chemistry of the environment (environmental chemistry).
These are the exact notes I used to ace my CIE Chemistry exam.
So, let’s dive straight in.
Chemical tests for water:
Is there any way to confirm the presence of water in a liquid?
Yes!
Here are the two methods you need to know about:
Anhydrous Cobalt(II) Chloride:
The dry Cobalt (II) Chloride paper changes colour from blue to pink with the addition of water.
If the colour remains unchanged, it shows the absence of water in the liquid.
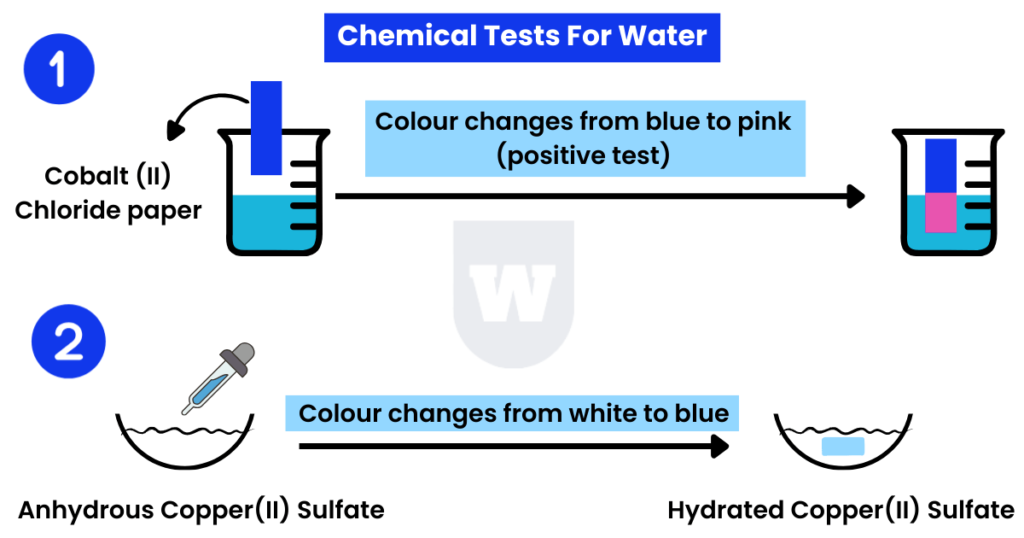
Anhydrous Copper(II) Sulfate:
Pure Copper (II) Sulfate, also called anhydrous Copper Sulfate, is white in colour.
When water is added to a Copper (II) Sulfate sample, the colour turns blue.
The change of colour from white to blue confirms the presence of water.
Here’s the chemical equation to show this:
Anhydrous Copper(II) Sulfate + Water ⇌ Hydrated Copper(II) Sulfate
CuSO4 (s) + 5H2O (l) ⇌ CuSO4.5H2O (s)
This takes us to the next topic.
Testing the purity of water:
The above tests only show the presence or absence of water.
They do not say anything about the purity of water.
So, how can we know whether water is pure or not?
Remember that pure substances have sharp melting at boiling points.
For example, pure water will have a boiling point of 100°C and a melting point of 0°C exactly.
But what if the water has impurities?
- Impure water will have a boiling point greater than 100°C.
- Impure water will have a melting point lower than 0°C.
So, here’s the takeaway.
Impurities generally increase the boiling point but decrease the melting point.
That’s why we use distilled water in practical Chemistry.
Why do we use distilled water?
Tap water is not a great choice for our lab experiments because it contains impurities.
On the other hand, distilled water leaves behind the chemical impurities.
So, what’s the big deal?
As a result of fewer chemical impurities in distilled water, we can carry out reliable and accurate experiments.
Remember this point for your practical exam.
Simple!
Naturally occurring substances in water:
We use water in our homes for drinking, washing, cooking, and cleaning.
This water is purified for us.
This is because water from natural resources, such as rivers and lakes, contains several other substances.
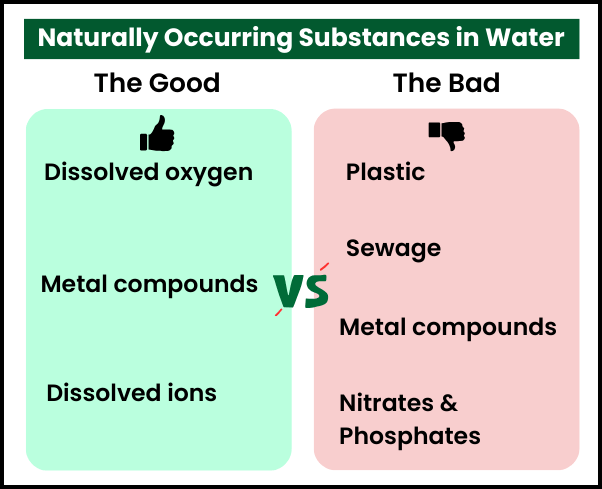
For example:
- Dissolved oxygen
- Dissolved ions
- Metal compounds
- Plastics
- Sewage
- Microorganisms
- Phosphates from fertilisers and detergents
- Nitrates from fertilisers
Let’s take a look at them.
The Good Ones:
- Dissolved oxygen in water is important for aquatic life and ecosystem health.
Let me explain.
Do you know how aquatic life, such as fish, get oxygen?
Exactly!
The dissolved oxygen in water allows aquatic organisms, like fish and invertebrates, to carry out respiration.
The microorganisms that break down organic matter in water also need oxygen.
The third reason dissolved oxygen in water is crucial is that it plays a key role in temperature regulation.
Just remember that warmer water holds less oxygen.
To summarise, the dissolved oxygen in water is crucial for:
- Respiration
- Decomposition
- Temperature regulation
This takes us to the next point.
- Metal compounds:
Metal compounds in water can be both beneficial and harmful.
But let’s talk about the good points first.
- Calcium: Important for bone and teeth formation, blood clotting, and nerve transmission.
- Sodium and Potassium: Crucial for muscle contraction and nerve transmission.
- Magnesium: For DNA or RNA synthesis.
- Iron: Formation of haemoglobin (oxygen transport).
Now, let’s talk about some dissolved substances that are harmful.
The Bad Ones:
- Metal compounds:
As I mentioned earlier, some metal compounds dissolved in water are harmful.
- Lead: Toxic and can cause severe health problems.
- Mercury: It contaminates fish and can damage the nervous system.
- Arsenic: Can cause health problems.
This takes us to the next point.
- Plastic:
Plastic harms aquatic life because ingested plastic can block the animal’s digestive system.
On the other hand, plastic can also leach harmful chemicals into water when exposed to sunlight.
So, you should know that not only does plastic contaminate water, but it also damages the habitat of aquatic life.
Easy?
- Sewage:
Remember that sewage contains harmful pathogens such as bacteria, viruses, and parasites.
So, what’s the big deal?
These harmful microbes can cause waterborne diseases such as cholera.
This takes us to the final point.
- Nitrates and Phosphates:
Cambridge wants you to know that nitrates and phosphates in water can lead to the deoxygenation of water.
But how?
What happens is that these nitrates and phosphates act as nutrients for algae and aquatic plants.
As a result, they grow rapidly.
When these algae and plants die, they are decomposed by bacteria, which consume oxygen from water.
This increased decomposition leads to oxygen shortage.
Interesting, isn’t it?
Treatment of Water:
Three important steps are carried out to treat water:
- Sedimentation and filtration to remove solids
- Use of carbon to remove taste and odour
- Chlorination to kill microbes
Let’s take a look at each of them.
Sedimentation:
First, water is pumped into sedimentation tanks and allowed to stand for some time.
Recall that the purpose of this is to remove solid particles from water.
To simplify the process, we add some chemicals into the tank that allow the solid particles (sediments) to settle.
These solid particles are then periodically removed. This way, we eliminate silt, clay, or organic matter.
The next step is filtration.
Filtration:
Solid particles not separated in the sedimentation tank are then removed through filtration.
The water is passed through several layers of sand, charcoal, and gravel to remove the remaining solid particles.
Take a look at the image below:
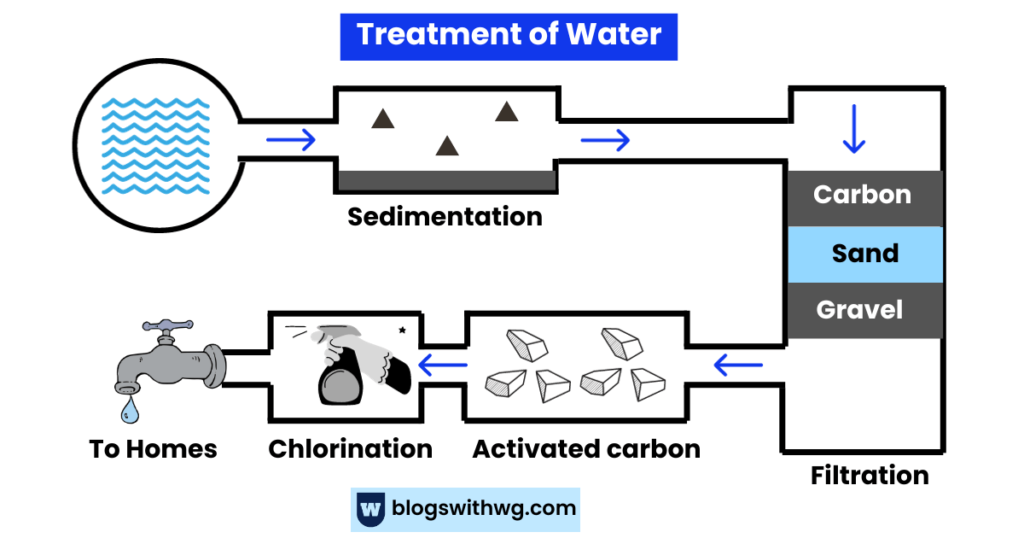
So, in this way, we get rid of smaller particles or impurities.
This takes us to the next step.
Removal of taste and odour:
We use activated carbon to remove the organic and inorganic compounds in water that cause unpleasant taste and odour.
In simple words, activated means that it is specially processed.
As a result, these unwanted particles get attached to the carbon.
Finally, we have chlorination.
Chlorination:
Chlorine acts as a disinfectant to kill microorganisms such as bacteria or viruses.
So, here’s a quick summary.
- Solid particles in the sedimentation tank are periodically removed.
- Water is passed through several layers of sand, charcoal, or gravel to remove the remaining solid particles (filtration).
- To remove taste or odour, we use activated carbon.
- Chlorine acts as a disinfectant to kill microorganisms.
This takes us to the next topic.
Fertilisers:
We will be talking about NPK fertilisers.
NPK fertilisers are those that contain nitrogen (N), phosphorus (P), and potassium (K).
These three macronutrients are extremely important for plant growth. Let me elaborate:
- Nitrogen (N): Role in chlorophyll production and is responsible for green foliage growth.
- Phosphorus (P): Role in root development.
- Potassium (K): Maintains the overall health of the plant.
But, here’s a problem.
Plants cannot take these nutrients directly.
That’s where ions in fertilisers come into action.
- Ammonium (NH4+) and nitrate ions (NO3-) are soluble nitrogen sources.
- Phosphate ions (PO43-) are a source of soluble phosphorus.
That’s why nitrates or ammonium salts are commonly used as fertilisers. Some examples are:
- Ammonium phosphate: (NH4)3PO4
- Ammonium nitrate: NH4NO3
- Potassium sulfate: K2SO4
This takes us to the next topic.
Composition of Air:
After discussing water and fertilisers, it’s time to discuss air.
Here’s what clean, dry air is composed of:
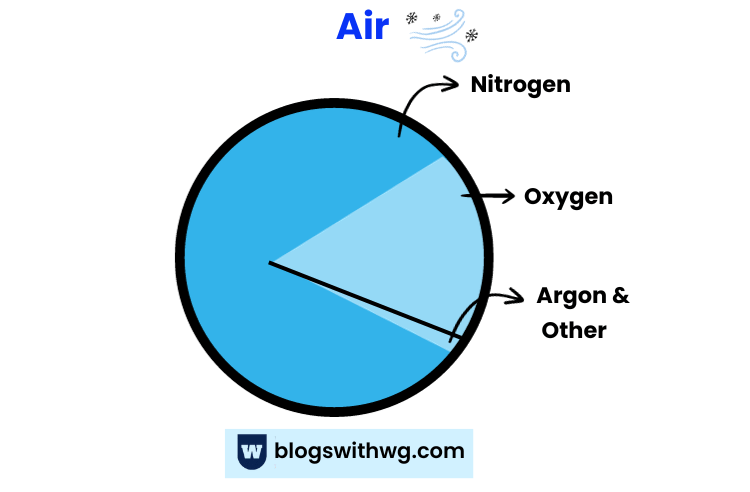
- Nitrogen: 78%
- Oxygen: 21%
- Argon: 0.9%
- Carbon dioxide, water vapours, and other noble gases make the rest.
Isn’t it interesting that Nitrogen is the most abundant naturally occurring gas in the atmosphere?
Once you understand this, it is important to learn about the sources of air pollution.
Air Pollution:
| Air Pollutant | Source |
|---|---|
| Carbon dioxide | Complete combustion of carbon-containing fuels |
| Carbon monoxide | Incomplete combustion of carbon-containing fuels |
| Methane | Released during the digestion processes of animals |
| Oxides of nitrogen | From vehicle engines by the combustion of nitrogen compounds in the fuel |
| Sulfur dioxide | Combustion of fossil fuels containing sulfur, such as coal and oil |
Now that we know about these air pollutants, it is time to know why we call them “pollutants.”
Adverse Effects of Pollutants:
- Carbon dioxide:
Although CO2 is a natural component of the Earth’s atmosphere, the increased concentration leads to adverse effects.
Let me explain.
Climate change.
Although it may not seem too big of a deal for you, it is.
Carbon dioxide gas traps heat and other greenhouse gases, leading to a rise in global temperature.
Rise in global temperature = Global warming.
Eventually, this results in climate change, severe heat waves, ecosystem disruption, and rising sea levels.
Now, let’s talk about carbon monoxide.
- Carbon monoxide:
It is a toxic gas.
What makes it extremely dangerous for humans is that it binds to haemoglobin.
As a result, the oxygen-carrying capacity of our blood decreases.
It also contributes to smog formation.
Here are some other:
| Pollutant | Effect |
|---|---|
| Particulates | These air pollutants consist of tiny, solid particles or liquid droplets suspended in air. They can penetrate deep into the lungs, causing respiratory problems or even cancer. |
| Methane | Higher levels of methane lead to increased global warming, which leads to climate change. |
| Oxides of nitrogen | Acid rain, photochemical smog, and respiratory problems. |
| Sulfur dioxide | Acid rain |
How is global warming caused?
You have already heard that greenhouse gases such as methane and carbon dioxide lead to global warming.
But how?
What happens is that the sun’s rays penetrate the atmosphere.
However, when that heat is reflected off the surface, it cannot escape back into space.
That’s the problem.
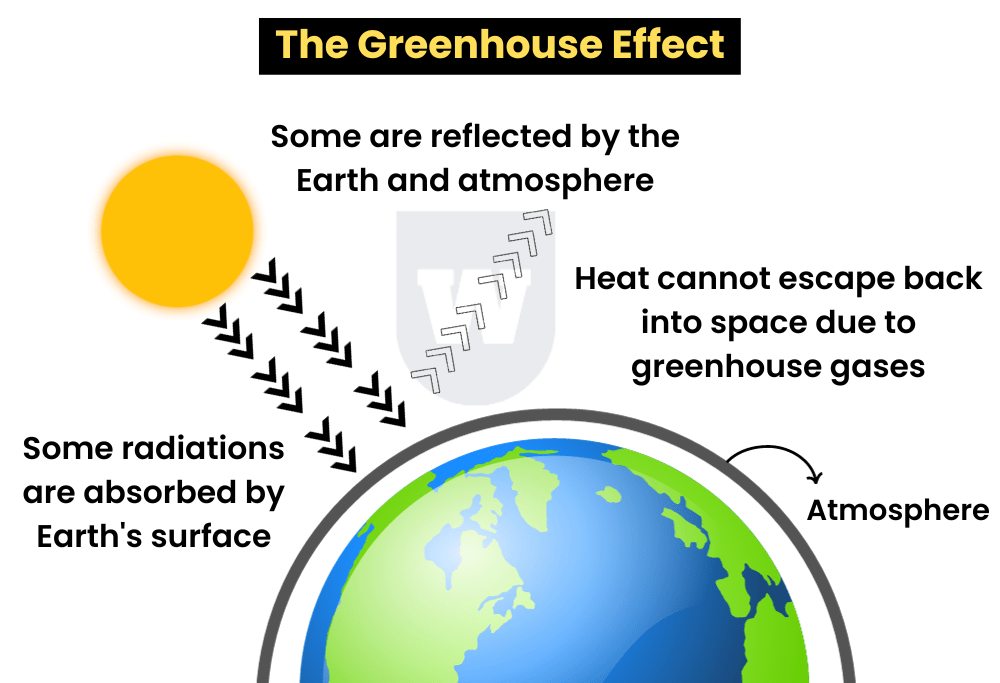
Greenhouse gases such as carbon dioxide and methane, produced by burning fossil fuels, trap this heat.
This is known as the greenhouse effect.
The heat that is not lost causes the global temperature to increase.
So, to summarise:
- Thermal energy is absorbed, reflected, and emitted.
- Reducing thermal energy loss to space makes the Earth warmer.
This takes us to the next point.
Dealing with climate change:
Here are a few steps that we can take to counter climate change:
(read the explanation, as it will help you develop your answers)
- Planting trees → Trees remove CO2 (a major greenhouse gas) from the atmosphere.
- Reduction in livestock farming → Methane and nitrous oxide are two greenhouse gases produced by rearing farmed animals.
Moreover, the conversion of forests to livestock ranches (deforestation) should be stopped.
- Decreasing use of fossil fuels → Fossil fuels, such as coal, oil, or gas, release large amounts of greenhouse gases when burned.
To solve this problem, investing in renewable energy to reduce the demand for fossil fuels is better.
The fewer fossil fuels burned, the fewer greenhouse gases emitted.
- Increasing use of hydrogen and renewable energy → As mentioned earlier, renewable energy is an alternative to fossil fuels.
Renewable energy sources around us, such as air, water, and heat from the sun, emit little to no greenhouse gases or pollutants into the air.
Unlike other fuels, hydrogen fuel does not release CO2 when burned.
Great, isn’t it?
How to deal with acid rain?
Let’s discuss some strategies to deal with acid rain.
Catalytic converters in vehicles:
You won’t be surprised that cars generate many gases and fumes.
To reduce the emission of gases, catalytic converters come into action.
Here’s how:
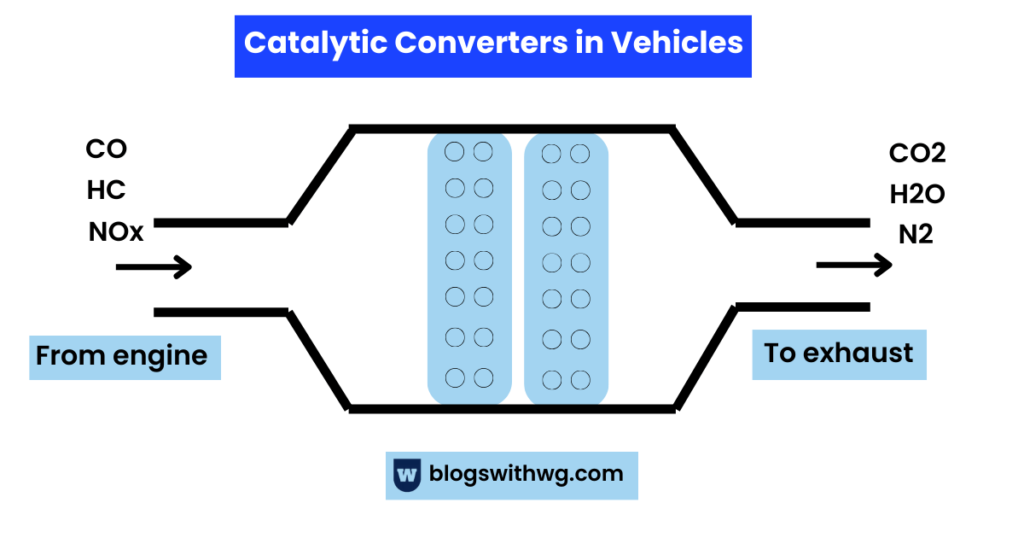
This part of the vehicle’s exhaust system converts harmful gases into harmless.
The chemicals and metal catalysts inside the converter cause chemical reactions that convert toxic pollutants into relatively harmless ones.
For example:
- Reduction reactions convert oxides of nitrogen to nitrogen.
- Oxidation reactions convert carbon monoxide to carbon dioxide.
Another way to deal with acid rain is flue gas desulfurisation with calcium oxide.
Flue Gas Desulfurisation:
We allow the acidic sulfur dioxide to react with basic calcium oxide.
As a result, we get calcium sulfite (CaSO3).
This is then oxidised to calcium sulfate. The summary is:
CaO (s) + SO2 (g) → CaSO3 (s)
2 CaSO3 (s) + O2 (g) → 2 CaSO4 (s)
This way, we can deal with acid rain by reducing sulfur dioxide.
Photosynthesis:
CIE also wants you to know about photosynthesis.
(the basics that we already know)
In the process, light energy is converted to chemical energy.
Wrapping Up:
Now, I turn it over to you.
Let me know if you have any questions regarding chemistry of the environment (environmental chemistry).
Here are some other CIE O Level Chemistry chapter notes:
Unit 2: Atoms, Elements and Compounds
Unit 6: Chemical Reactions
Unit 7: Acids, bases, and salts
Unit 9: Metals
Unit 12: Experimental Techniques and Chemical Analysis
Happy learning!
