You will love this comprehensive guide if you want to learn about atoms, elements, and compounds.
These are the exact notes I used to ace my Chemistry exams.
So, let’s dive in to explore the fascinating world of atoms.
Difference Between Elements, Compounds, and Mixtures:
Take a look at the periodic table.
Notice copper, zinc, iron, and sulfur? These are elements.
An element is a pure substance that cannot be broken down into two or more simpler substances.
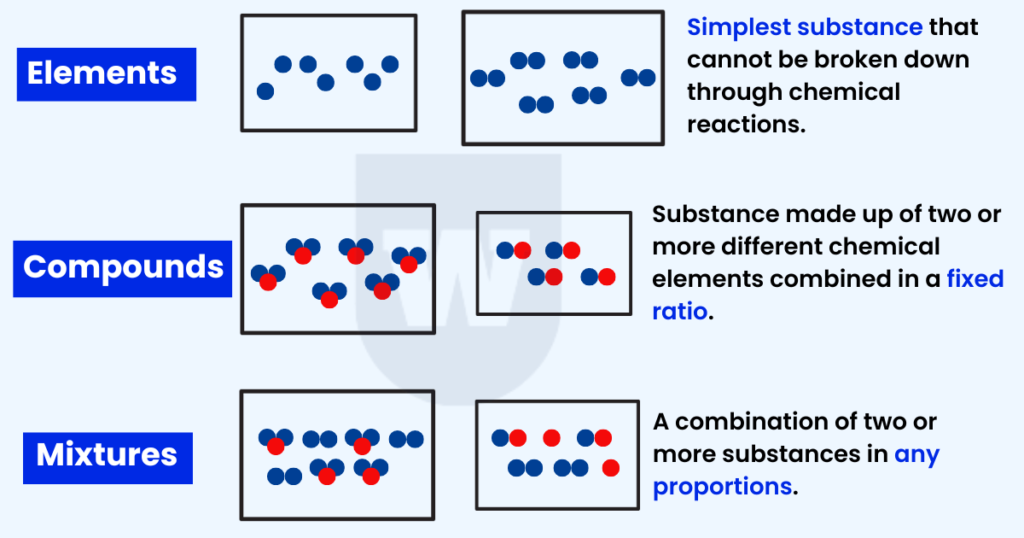
Remember that each element contains only one type of atom.
What’s a compound?
We all know that water is H2O. Every water molecule contains two atoms of hydrogen (H) joined to a single atom of oxygen (O).
That’s what a compound is.
A compound is a pure substance containing two or more elements chemically combined in a fixed ratio.
Ammonia (NH3), carbon dioxide (CO2), and sucrose (C12H22O11) are some other compounds.
What else do you notice in a compound?
- Unlike an element, a compound can be broken down by chemical methods such as thermal decomposition or electrolysis.
- The physical and chemical properties of a compound are different from those of its constituent elements.
- A chemical reaction takes place when a compound is formed.
Now, let’s talk about mixtures.
What is air?
You will say that air is a mixture of gases like nitrogen, oxygen, carbon dioxide, argon, neon, etc.
Exactly!
A mixture comprises two or more substances (in any proportion) that are not chemically combined.
Plus, you can separate a mixture by physical processes, such as using a magnet or filtration and distillation.
For example, we can remove salt from seawater (mixture).
Here are some other properties of mixtures.
- The chemical properties of a mixture are identical to those of the individual substances that make it up.
- No chemical reaction occurs when a mixture is formed. And usually, there are no (unnoticeable) energy changes.
It is time to move on to the next point when you know this.
Atomic Structure:
Atoms are not as simple as they sound.
Atoms are made up of fundamental particles like protons, neutrons, and electrons.
If we talk about the structure of an atom, we have protons and neutrons making up the nucleus.
The electron shells outside the nucleus contain electrons (negatively charged). Take a look:
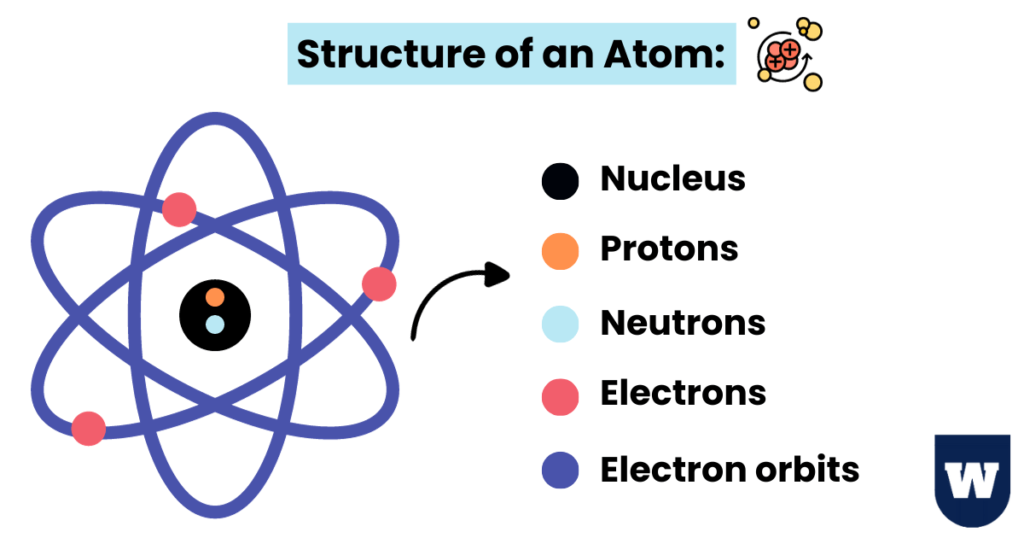
You should learn that protons and electrons are charged particles, whereas neutrons are neutral.
This means that the atom is an electrically neutral entity.
Here are the relative charge and masses of these three particles for you to learn:
| Charge | Mass (amu) | Location | |
|---|---|---|---|
| Proton | +1 | 1 | Nucleus |
| Neutron | 0 | 1 | Nucleus |
| Electron | -1 | 0 | Orbitals |
This takes us to the next point.
Proton and Mass Number:
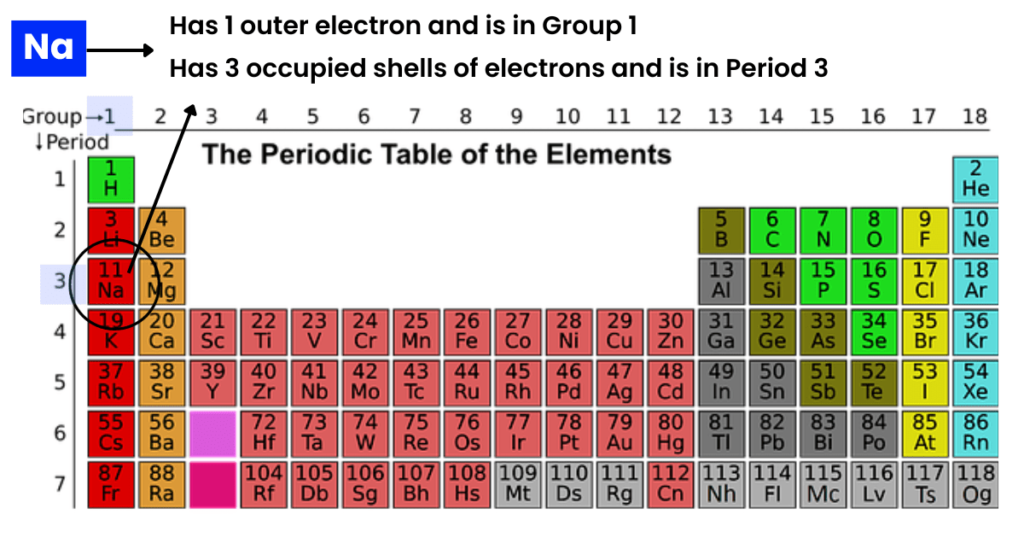
Take a look at sodium (Na) in the periodic table.
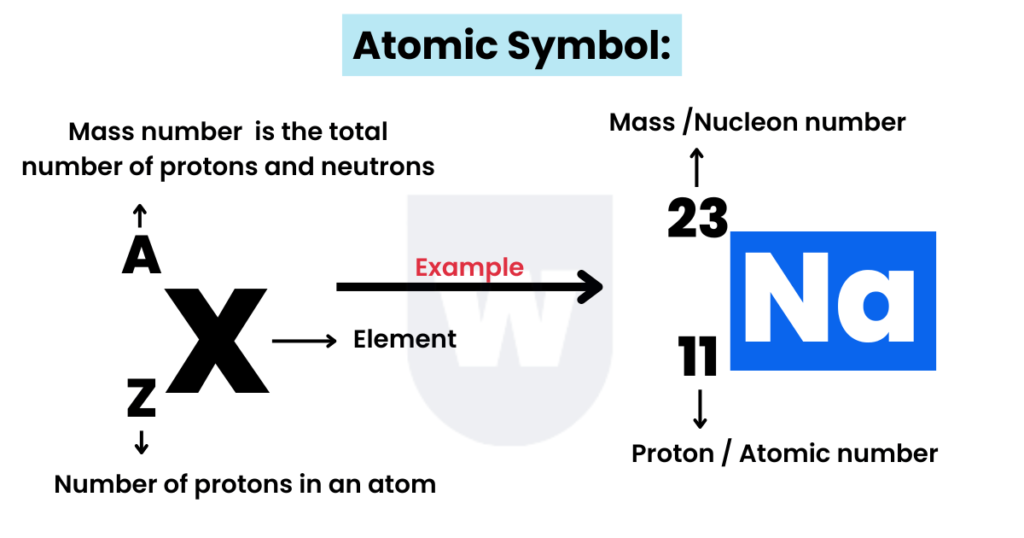
What do you notice?
- Atomic (proton) number: Number of protons in an atom, specifically in a nucleus.
Let’s have a look at sodium (Na).
As you can see above, it has 11 protons. So, its atomic number is 11.
Important Note: As an atom is electrically neutral, the number of electrons equals the number of protons.
Therefore, the number of electrons in a sodium atom is also 11.
Na: Protons = Electrons = 11
Now, let’s talk about the mass number.
- Mass (nucleon) number: Number of nucleons (sum of protons and neutrons) in an atom’s nucleus.
For example, sodium has 11 protons, and we know the mass number of sodium is 23. This means that sodium has 12 neutrons (23-11).
This takes us to the next topic.
Electronic Configuration:
CIE wants you to know about the electronic configuration of elements and their ions with proton numbers 1 to 20.
We know what “proton number” is, right? But what is electronic configuration?
To answer this question, we must first look at the arrangement of electrons in the atom. Here’s what you need to know:
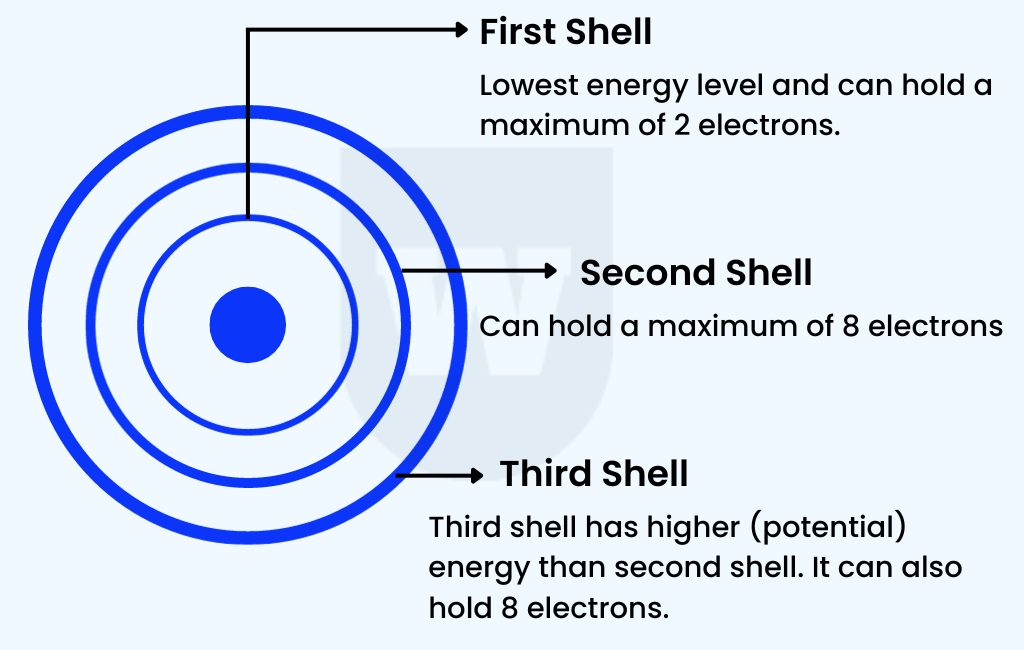
- The electrons in an atom move around the nucleus in regions known as electron shells.
Each electron shell corresponds to a specific energy level and can hold only a certain fixed number of electrons.
For example:
- The first shell (closest to the nucleus) has the lowest energy level and can hold a maximum of 2 electrons.
The first shell is always filled first.
- The second and third shells have increasing energy levels.
This means that the third shell has more energy than the second. They can hold a maximum of 8 electrons each and are filled in order.
So, when the second shell is fully filled, we will move on to the third shell.
Simple! Note that the fourth shell can hold up to 18 electrons.
Electronic Configuration of the first 20 elements:
Take a look at the table below:
| Element | Atomic Number | Electronic Configuration |
|---|---|---|
| Hydrogen | 1 | 1 |
| Helium | 2 | 2 |
| Lithium | 3 | 2, 1 |
| Beryllium | 4 | 2, 2 |
| Boron | 5 | 2, 3 |
| Carbon | 6 | 2, 4 |
| Nitrogen | 7 | 2, 5 |
| Oxygen | 8 | 2, 6 |
| Fluorine | 9 | 2, 7 |
| Neon | 10 | 2, 8 |
| Sodium | 11 | 2, 8, 1 |
| Magnesium | 12 | 2, 8, 2 |
| Aluminium | 13 | 2, 8, 3 |
| Silicon | 14 | 2, 8, 4 |
| Phosphorus | 15 | 2, 8, 5 |
| Sulfur | 16 | 2, 8, 6 |
| Chlorine | 17 | 2, 8, 7 |
| Argon | 18 | 2, 8, 8 |
| Potassium | 19 | 2, 8, 8, 1 |
| Calcium | 20 | 2, 8, 8, 2 |
Are you confused about how to write electronic configuration?
Let’s take sodium as an example.
Sodium has 11 electrons. The first two are filled in the first shell, which leaves 9 electrons.
8 of these 9 electrons are filled in the second shell, and the 1 electron remaining goes to the third shell.
So, we write its electronic configuration as 2, 8, 1
This takes us to the next topic.
The Periodic Table:
As the syllabus states, we must understand three important things about the periodic table:
- The group VIII elements are called noble gases. Why noble?
Well, they’re basically unreactive. Why unreactive? That’s because all of the noble gases have a full outer shell.
For example, the electronic configuration of Argon is 2, 8, 8.
They do not have free electrons to form bonds. So, they are unreactive.
- The outer shell electrons are equal to the group number in Groups I to VII.
For instance, have a look at sodium, a group I element.
How many outer shell electrons? One.
Still want to be sure? Fair enough.
Observe Chlorine, group number VII. Its electronic configuration is 2,8,7. So, it has seven outer shell electrons.
Interesting, isn’t it?
- The number of occupied electron shells is equal to the period number.
There are seven periods in the periodic table. If an element is present in the third period, it will have three occupied electron shells.
Observe both sodium (2,8,1) and magnesium (2,8,2).
Both are members of the third period and have three occupied electron shells.
Now that we are done with atomic structure and the periodic table, it’s time to learn about isotopes.
Isotopes:
Isotopes are atoms of the same element with the same number of protons and electrons but different numbers of neutrons.
Take a look at the image below:
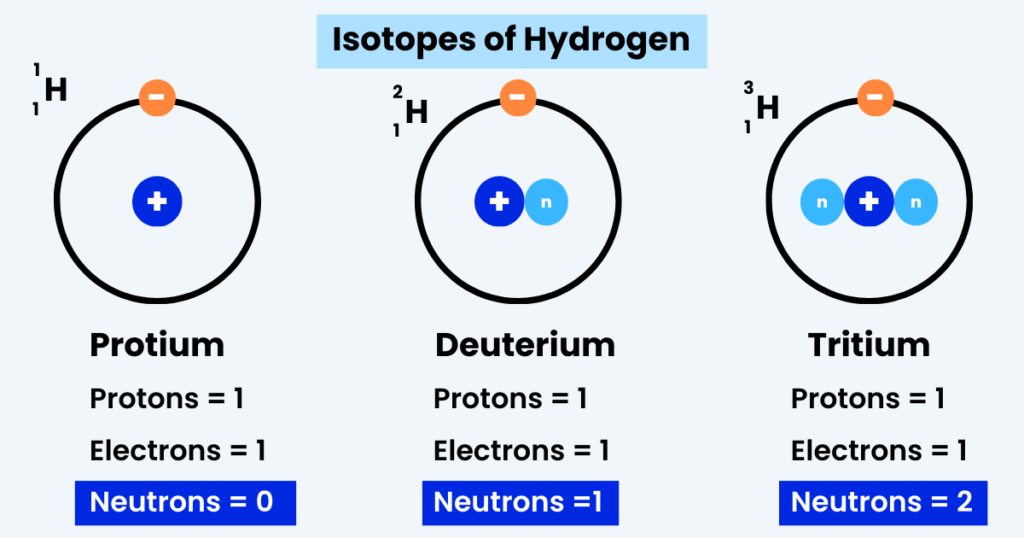
What do you notice?
- Protium has one proton but no neutron.
- Deuterium has a neutron along with a proton.
- Tritium has two neutrons and a proton.
The idea is that neutrons are NOT the same in isotopes.
Now, let’s talk about the physical and chemical properties of isotopes.
There’s one thing I want you to remember.
Chemical properties always depend upon the number of electrons. Therefore:
- Isotopes have the same chemical properties because of the same number of protons and electrons (the same electronic configuration).
- Isotopes do NOT have the same physical properties.
This is because the number of neutrons in an atom contributes significantly to its mass.
Before moving ahead, take a look at the image below:
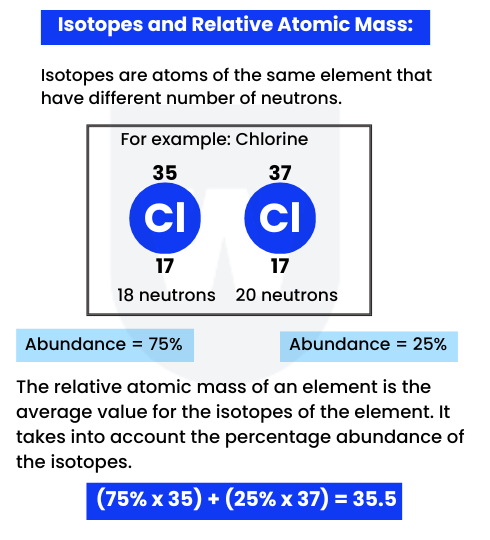
If you look at the mass number of Chlorine from the periodic table, it is 35.5.
Why not 35 or 36?
Well, this is because isotopes exist in different percentages. Therefore, we find the relative atomic mass (as shown in the image above).
With this, it is time to move on to the next topic.
Ion and Ionic Bonds:
Here are all the key points you need to know:
- Metals lose electrons to form positive ions (cations).
- Non-metals gain electrons to form negative ions (anions).
Remember that this happens so they become stable by completing their outer shell.
These positive and negative ions are linked by an ionic bond.
Note: An ionic bond is a type of linkage formed between oppositely charged ions due to their electrostatic attraction.
Here’s how an ionic bond is formed between a sodium and chloride ion.
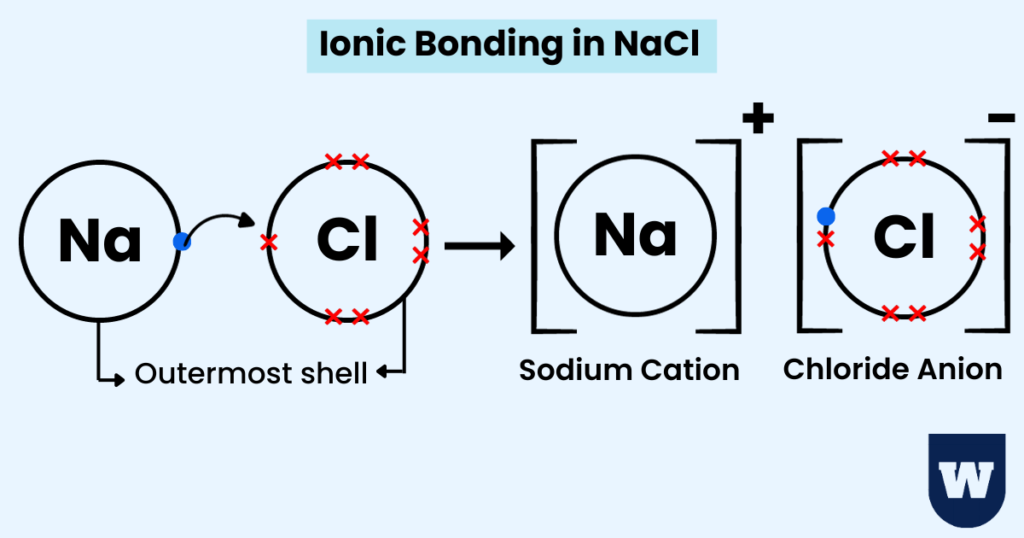
- A sodium atom loses its outer electron (to the chlorine atom). A sodium cation is formed.
- A chlorine atom gains an electron from sodium. A chloride anion is formed.
- Electrostatic attraction (ionic bonds) holds the sodium and chloride ions together.
An ionic compound, sodium chloride, is formed.
To learn more about ionic bonding and for some questions, you can check out these:
Ionic Bonding Explained in Detail
This takes us to the next point.
Properties of ionic compounds:
- Ionic compounds have high melting and boiling points.
Do you know that the melting point of Sodium Chloride (NaCl) is 801°C and its boiling point is 1413°C?
Pretty high, isn’t it?
Here is the reason for that.
We learned that ionic compounds have strong electrostatic forces of attraction between oppositely charged (positive and negative) ions.
Therefore, a very high temperature is required to overcome these strong forces of attraction.
You should also remember that ionic compounds have a giant lattice structure. So, a lot of heat energy is required to break this structure.
Let’s move on to the next point.
- Ionic compounds conduct electricity in molten or aqueous solutions.
Do you know that ionic compounds do NOT conduct electricity in the solid state?
To conduct electricity, you should have free-moving ions or electrons.
So, ionic compounds in a solid state do not have free-moving (mobile) ions or electrons to conduct electricity.
But in an aqueous solution, they have free-moving ions.
In the same way, molten (melted) compounds will also conduct electricity due to the presence of electrons.
This takes us to the next topic.
Covalent Bonds:
Can two non-metals form bonds with each other?
YES!
A covalent bond is formed when a pair of electrons is shared between two atoms, leading to a noble gas electronic configuration (stable).
A covalent bond can be formed between:
- Atoms of the same element
- Atoms of different element
To better understand this, take a look at the image below:
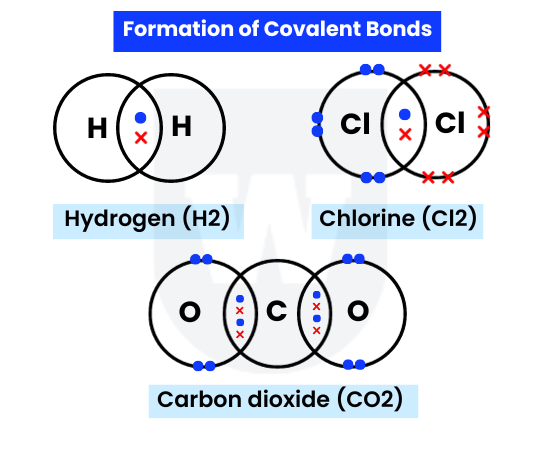
Here’s how covalent bonds form in hydrogen (H2):
- A hydrogen atom has one outer electron. It needs one more electron to attain a stable duplet electronic configuration.
- Another hydrogen atom shares its outer electron with that hydrogen atom.
- A pair of electrons is shared between the two hydrogen atoms, forming a single covalent bond (one shared pair of electrons).
In this way, both hydrogen atoms attain a stable duplet electronic configuration.
Take a look at the above image again to observe CO2.
What do you notice?
- The carbon atom has four outer electrons. It needs four more electrons to attain a stable octet electronic configuration.
- Each oxygen atom has six outer electrons. It needs two more electrons to attain a stable octet electronic configuration.
- A carbon atom shares its four electrons with two oxygen atoms, forming two double covalent bonds.
Note that a double covalent bond means two shared pairs of electrons, meaning four electrons are shared in total.
Earlier, we discussed the structure of ionic compounds. What’s the case with covalent compounds?
Covalent compounds usually form:
- Simple molecular structures
- Giant molecular structures
Most covalent substances are simple molecules, such as Bromine.
These simple molecular structures have the following properties:
- Low melting and boiling points due to weak intermolecular forces.
- Insoluble in water but soluble in organic solvents.
- Most substances with simple molecular structures do not conduct electricity.
Now, let’s discuss giant molecular structures.
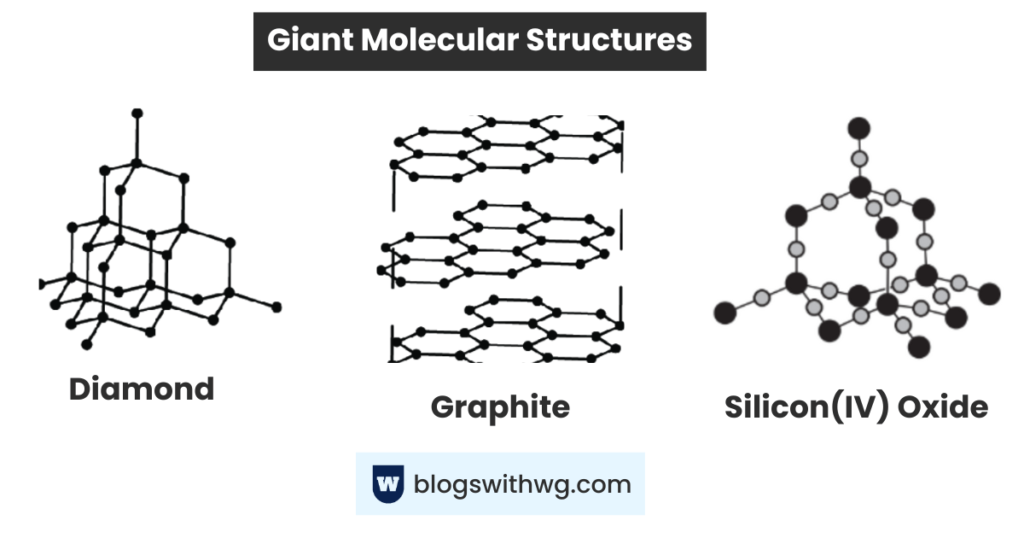
Giant Molecular Structures:
Some covalent substances form a giant network of atoms covalently bonded to each other.
These substances have a high melting and boiling point. Plus, they do not conduct electricity (except graphite).
Let’s take a look at some of them:
Graphite:
- High melting and boiling point.
Each carbon atom is bonded to three other carbon atoms, which are bonded to three other carbon atoms.
This results in a continuous layer of hexagons, which is difficult to break.
- Graphite is soft and slippery.
The layers of carbon atoms are loosely attached to each other due to weak intermolecular forces.
These layers can slide over each other when force is applied. This is also why graphite can be used as a lubricant.
- Conducts electricity.
Each carbon atom has one outer electron not used to form covalent bonds; these electrons can move freely along the layers and are said to be delocalized.
These free-moving electrons help graphite conduct electricity.
Due to its electrical conduction property, graphite is used as an electrode.
Diamond:
- It is hard and has a high melting and boiling point.
Each carbon atom is bonded to four other carbon atoms, which are bonded to four other carbon atoms, forming a three-dimensional structure.
This hardness makes diamonds a widely used material in making cutting tools.
- Does not conduct electricity.
All the outer electrons of carbon atoms are used in bonding. So, there are no free-moving electrons to conduct electricity.
This takes us to the next point.
Silicon(IV) Oxide:
- It has a high melting and boiling point.
Here’s what you need to know.
(tetrahedral macromolecular structure)
Silicon (IV) oxide has a similar structure to diamond. Diamond has a carbon atom bonded to four other carbons.
Similarly, silicon (IV) oxide has a silicon atom bonded to four other oxygen atoms, forming a three-dimensional structure.
Like diamond, it also does not conduct electricity as no free electrons are available.
This takes us to metallic bonding.
Metallic Bonding:
Let’s talk about metals.
Do they form simple molecular structures? Do they form giant molecular structures?
No!
Metals have a lattice structure, and here’s what you need to know:
- Metal atoms lose their outer electrons to become positively charged ions.
- The outer electrons become delocalized and move freely between the metal ions.
- Hence, the metal lattice structure is known as a “lattice of positive ions surrounded by a sea of mobile electrons.”
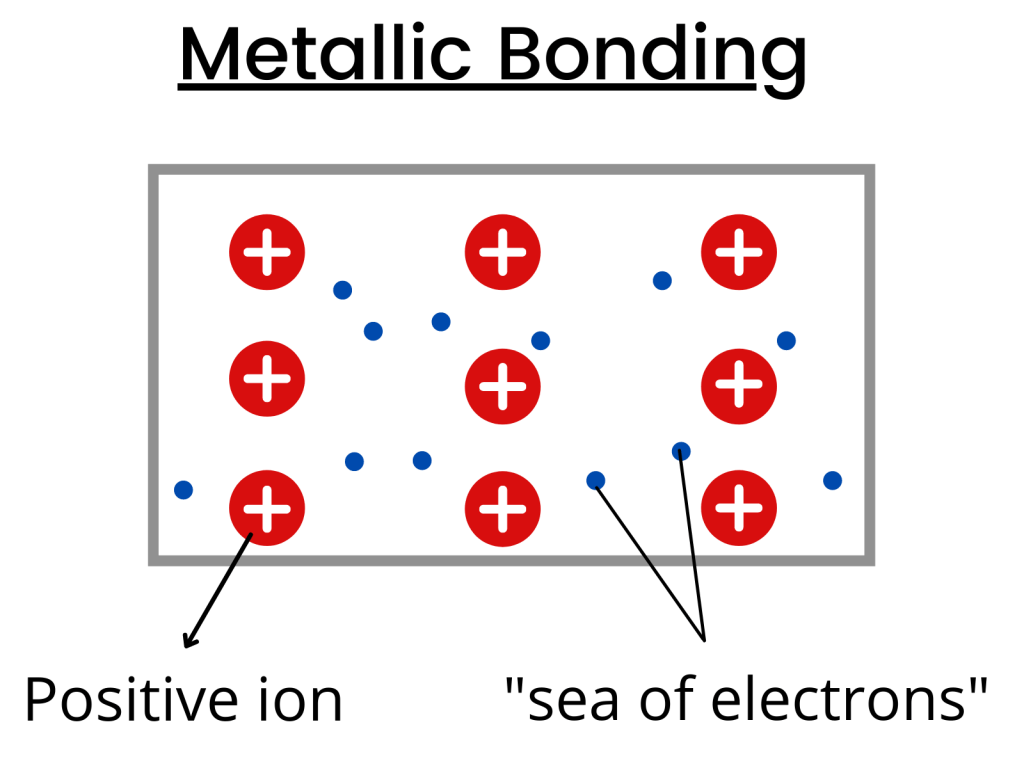
Apart from being good conductors of heat and electricity, they also have high density, melting, and boiling points.
Finally, they are malleable (pressed into different shapes without breaking or cracking) and ductile (can be drawn into a thin wire).
Wrapping Up:
Now, I turn it over to you.
If you have any questions regarding “atoms, elements and compounds,” do let me know.
Here are some other CIE O Level Chemistry chapter notes:
Unit 6: Chemical Reactions
Unit 7: Acids, bases, and salts
Unit 9: Metals
Unit 10: Chemistry of the Environment
Unit 12: Experimental Techniques and Chemical Analysis
Happy learning!
